Figures & data
Figure 1 CRMP-2 signaling cascade: a novel role for CRMPs in Ca2+ channel regulation and transmitter release. Extracellular signals, such as extracellular matrix, growth factors and guidance cues (semaphorin 3A) activate Neuropilin-1/Plexin A receptors on membranes.Citation7 A battery of kinases, including RhoK, Cdk5 and GSK-3β phosphorylate CRMPs. Phosphorylated CRMPs have a reduced affinity to tubulin and other interacting molecules and lose their positive effect on axon elongation, thereby causing growth arrest and growth cone collapse. In contrast, non-phosphorylated CRMPs bind strongly to tubulin heterodimers to promote microtubule assembly and Numb-mediated endocytosisCitation30 thereby promoting axon elongation and branching.Citation7 In addition to these classically defined roles for CRMPs, our results suggest that CRMPs (assuming both phosphorylated and non-phosphorylated forms) bind to cytoplasmic loops of the Ca2+ channel and increase their insertion into the membrane, resulting in an increased current density.Citation1 This increase culminates into an increase in the release of the excitatory transmitter glutamate.Citation1 Interestingly, CRMP-2 overexpression increases synapse size not number.Citation1 This suggests that CRMP-2 regulation of transmitters may occur via a direct effect on CaV2.2 or through an effect on changes in synaptic vesicle machinery and release probabilities. Increased synaptic transmission is likely to contribute to synaptic plasticity.
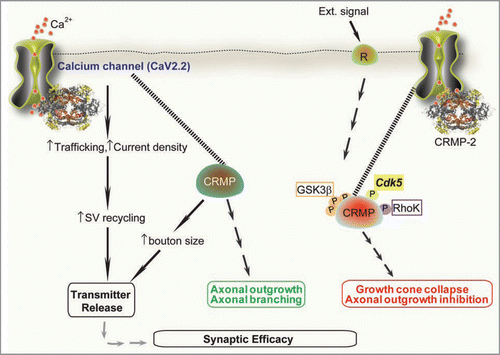
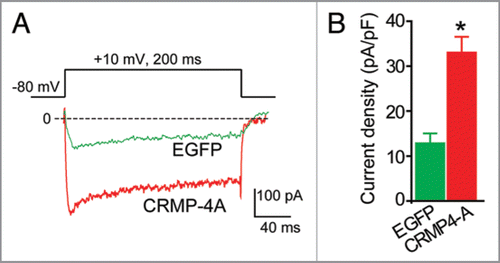
Figure 2 CRMP-4 enhances Ca2+ current density in hippocampal neurons. (A) Exemplar current traces obtained from a cell transfected with EGFP and CRMP-4-EGFP evoked by 200-ms steps to +10 mV applied from a holding potential of -80 mV, as shown in the voltage protocol above the traces. Bath solutions contained 1 µM TT X, 10 mM TE A and 1 µM Nifidepine to block Na+, K+ and L-type voltage-gated Ca2+ channels, respectively. (B) Peak current density (pA/pF) measured at +10 mV for EGFP (n = 8) or CRMP-4a-EGFP (n = 8) transfected neurons. *p < 0.05 versus EGFP (One-way ANOVA).