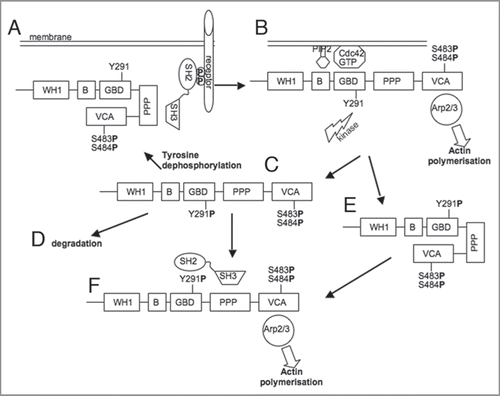
Figures & data
Table 1 Stimuli inducing WASp phosphorylation in cells