Figures & data
Figure 1 Actin and the synaptic vesicle cycle. Synaptic vesicles are tethered in the reserve pool (RP) by a network of actin filaments. Small GTPases, such as RhoA and Rac1, control the state of actin polymerization by governing pathways that involve phosphorylation/dephosphorylation of different actin-binding proteins. The WASP protein and the Arp2/3 complex promote the actin filament nucleation process necessary to build actin tracks for vesicles trafficking. Vesicles are transported to the active zone along actin filaments by myosin V, which also contributes to vesicles docking via its binding to syntaxin1. Primed vesicles are part of the ready releasable pool (RR P). Ca2+ influx and the assembly of the SNARE ternary complex formed by the α-helices of VAMP2, SNAP25 and syntaxin1, trigger vesicle exocytosis at the active zones plasma membrane. Therefore, actin regulates priming and fusion, directly affecting neurotransmitter release. In addition it constitutes the tracks that guide recycling vesicles back to the RP.
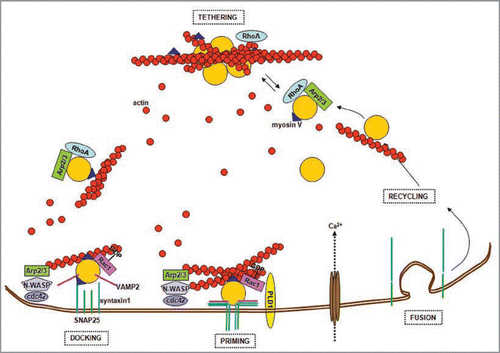
Figure 2 Hypothetical model of α-synuclein action on actin dynamics. At synaptic terminals α-synuclein is associated with synaptic vesicles. At rest, when [Ca2+]c is around 100 nM, α-synuclein binds preferentially actin monomers, sequestering them and slowing down the polymerization of microfilaments. When [Ca2+]c rises upon cell stimulation, α-synuclein affinity for actin monomers decreases and binding to filaments prevails, shifting the balance toward polymerization. This series of events is expected to facilitate the maintenance of vesicles in the RP and, at the same time, to favour the formation of the tracks and the clustering of molecules necessary for vesicles docking and fusion to take place.
![Figure 2 Hypothetical model of α-synuclein action on actin dynamics. At synaptic terminals α-synuclein is associated with synaptic vesicles. At rest, when [Ca2+]c is around 100 nM, α-synuclein binds preferentially actin monomers, sequestering them and slowing down the polymerization of microfilaments. When [Ca2+]c rises upon cell stimulation, α-synuclein affinity for actin monomers decreases and binding to filaments prevails, shifting the balance toward polymerization. This series of events is expected to facilitate the maintenance of vesicles in the RP and, at the same time, to favour the formation of the tracks and the clustering of molecules necessary for vesicles docking and fusion to take place.](/cms/asset/f371b046-6254-40d5-9bee-d9ba185bdc80/kcib_a_10910964_f0002.gif)