Figures & data
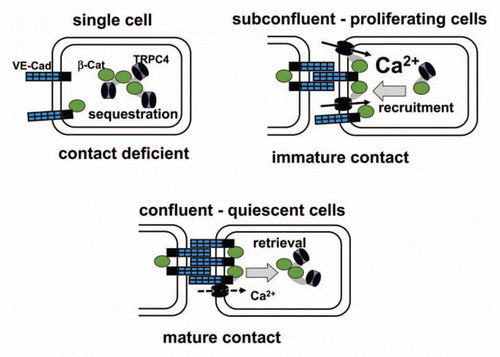
Figure 1 Proposed model of phenotype-dependent TRPC4 function in growth factor-stimulated endothelial cells via interaction of the channel with β-catenin and targeting into cell-cell contacts. TRPC4 is for a large part sequestered in intracellular compartments and unavailable for Ca2+ signalling in single cells (contact deficient; upper left). By contrast, formation of immature cell adhesions promotes surface targeting of β-catenin-TRPC4 complexes and enables further recruitment of channels into the plasma membrane and Ca2+ entry function (immature contact; upper right). Once mature barriers are formed (mature contact; lower), TRPC4 resides for a large part in junctional complexes that are rapidly retrieved from the cell surface during growth factor stimulation and are barely available for contribution to global Ca2+ signaling.