Figures & data
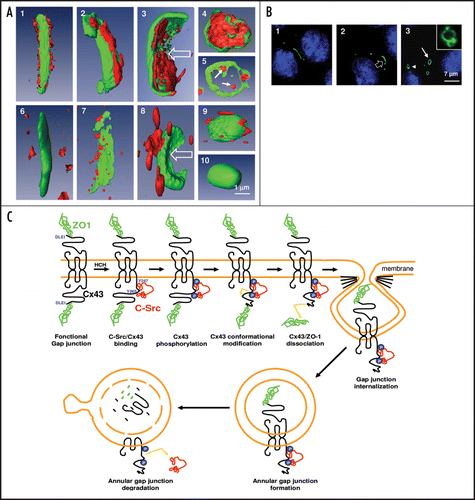
Figure 1 (A) Carcinogen exposure induces gap junction plaque internalization that is associated with the increased interaction of c-Src with Cx43, decreased association between ZO-1. The three-dimensional Amira analysis of the altered interactions between Cx43 (green fluorescence) and ZO-1 (red fluorescence) during gap junction plaque internalization is summarized in upper panels. The lower panels illustrate the modified association between c-Src (red fluorescence) and Cx43 (green fluorescence) during the same time-period. Panel A5 represents the internal annular gap junction degradation of ZO-1 and panel A10 illustrates the disappearance of c-Src from this structure at the same period. (B) Identification of the different phases of gap junction plaque endocytosis analyzed by meaning of Cx43 tagged with green fluorescent protein (GFP). Left: Cx43-GFP gap junction plaque observed between two adjacent cells. Middle: invagination of the structure within one cell. Right: formation of an annular gap junction, which is present at the beginning of the endocytosis phase near the plasma membrane (arrow) and afterwards in the nuclear region around the nucleus (arrowhead). The inset represents a degradative form of annular gap junction. (C) Schematic representation of the molecular mechanisms by which ZO-1 and c-Src interact with Cx43 during gap junction plaque endocytosis. In control cells, Cx43 is associated with ZO-1. After HCH exposure, activated c-Src bind to Cx43 and induced Cx43 phosphorylation on tyrosine residues as suggested.Citation30 These modifications could be involved in Cx43/ZO1 dissociation in the gap junction side that binds c-Src, and induce gap junction internalization on the side of the gap junction plaque in which Cx43/ZO-1 association was probably affected in concert with other Cx43 protein partners (clathrin, actin…). Then annular gap junctions that contain ZO-1 inside and c-Src outside of the vesicle are degraded during annular gap junction trafficking from the plasma membrane to lysosomal area close to nuclei giving rise to disappearance of both proteins from their original position.