Figures & data
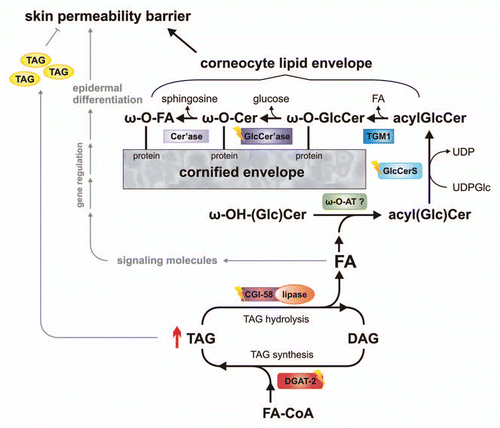
Figure 1 Schema depicting the role of epidermal triacylglycerol (TAG) metabolism in skin permeability barrier development. Acyl-CoA:diacylglycerol acyltransferase-2 (DGAT-2) transfers an activated fatty acid (FA-CoA) onto diacylglycerol (DAG) to generate TAG. These TAGs are hydrolyzed by a lipase, co-activated by comparative gene identification-58 (CGI-58). TAG-derived FAs are then activated and transferred by an unknown ω-(O)-acyltransferase (ω-O-AT?) onto ω-OH-(glucosyl)ceramides (ω-OH-(Glc)Cer). Acylceramides (acylCer) are glucosylated by uridine diphosphate glucose:ceramide glucosyltransferase (glucosylceramide synthase, GlcCerS). Generated acylglucosylceramides (acylGlcCer) are then covalently bound to proteins of the cornified envelope by a transesterification reaction (transglutaminase 1, TGM1), deglucosylated (β-glucocerebrosidase-GlcCer'ase) and hydrolyzed (ceramidase, Cer'ase). TAG-derived FAs may also act as ligands for nuclear hormone receptors, activating transcriptional regulators involved in epidermal differentiation. Excessive epidermal TAG accumulation (as indicated by ↑) may lead to lamellar/nonlamellar phase separation and permeability barrier dysfunction. Defects in protein function (as indicated by flashes) result in ichthyosiform phenotypes.