Figures & data
Figure 1. Small RNA-generating loci are suppressed in nrpe mutants. (A) Small RNA size profiles in wild-type (Col) and a null mutant of NRPE1 (nrpe) replicate libraries, normalized to the percentage of 21 nt abundance in wild-type libraries. For each size class, small RNA abundance (excluding structural RNA) was calculated as a percentage to the sum of abundances of total genome-matched reads. (B) Averaged percentage of abundances in small RNA size classes, calculated from data in (A); “Col avg” or “nrpe avg” indicate the averaged values for each set of three libraries. (C) Number of clusters impacted in the nrpe mutant, based on summed small RNA abundances per cluster. For each cluster, we compared the average of small RNA HNA of three Col libraries to the average of small RNA HNA of three nrpe libraries. The ratio of Col vs. nrpe is used when the HNA of Col libraries is greater than that in nrpe libraries, while the ratio of nrpe vs. Col is used when the HNA of nrpe libraries is greater than that in Col libraries (shown as negative values). The inset graph (note the reduced y-axis) expands to the full range of the fold differences to explain the high value of the “≥ 10” column; the basis for the high “≥10” bar is a very long tail of low frequency clusters highly impacted in nrpe. (D) Genic vs. non-genic and repeat- vs. non-repeat-associated characteristic of RNAP V-dependent small RNA clusters based on the TAIR version 9 annotations. RNAP V-dependent (“RNAP V-dpt”) and RNAP V-independent (“RNAP V-indpt”) clusters were defined as described in text. A total of 11,667 small RNA-generating, 2,201 RNAP V-dependent and 7,680 RNAP V-independent clusters were analyzed.
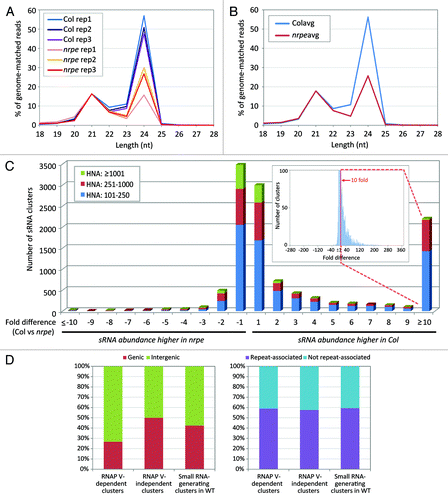
Figure 2. Genome-wide distributions of RNAP V-dependent small RNA-generating loci. Upper panel, the distribution of small RNA-generating loci and small RNA abundance in wild-type along chromosome 1 with bar height indicating the sum of HNA in Col libraries; red bars indicate HNA greater than 500; full-height bars are HNA between 100 to 500, and shorter bars are HNA < 100. The chromosome is illustrated below in gray, with approximate pericentromeric regions based on centromeric staining data marked with green bars.Citation51,Citation62 Middle panel, differentially-expressed RNAP V- dependent small RNA-generating clusters; full-height bars in black are clusters with reduction relative to Col of between 10- to 50-fold; red bars indicate clusters with fold reduction > 50. Lower panel, the density of RNAP V-dependent clusters across chromosome 1, plotted as the percentage of number of clusters in 1 Mb windows over the total number of RNAP V-dependent clusters mapped to chromosome 1. The distribution of small RNA-generating clusters and NRPD1(RNAP IV)- and RDR2-dependent clusters were plotted for comparison. Data for chromosomes 2 to 5 are shown in Figure S2.
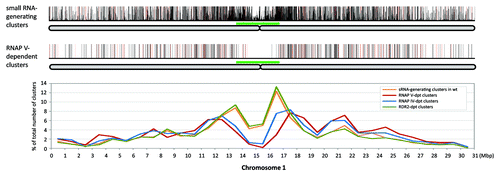
Figure 3. SINEs are overrepresented in nrpe-suppressed siRNA-generating loci. (A) Classes of repetitive sequences represented in RNAP V-dependent small RNA-generating clusters. RNAP V-dependent and RNAP V-independent clusters are defined as described in the legend. Repetitive sequences are identified based on Arabidopsis TAIR9 repeat annotation. Percentage of repeat-annotated clusters to the numbers of RNAP V-dependent or RNAP V-independent loci is shown. Change of repeat class representation is calculated by the ratio of its percentage in RNAP V-dependent vs. RNAP V-independent cluster (in red) or by the ratio of its percentage in RNAP V-independent vs. RNAP V-dependent cluster (in blue), which indicates whether the repeat class is over-represented or under-represented in RNAP V-dependent loci, respectively. (B) Overrepresentation of SINEs in RNAP V-dependent clusters. Number of SINE-annotated clusters is plotted against the fold difference in small RNA abundance between wild-type and nrpe libraries. Number of RC/Helitron-annotated clusters, another repeat class that is overrepresented in RNAP V-dependent clusters, is also plotted.
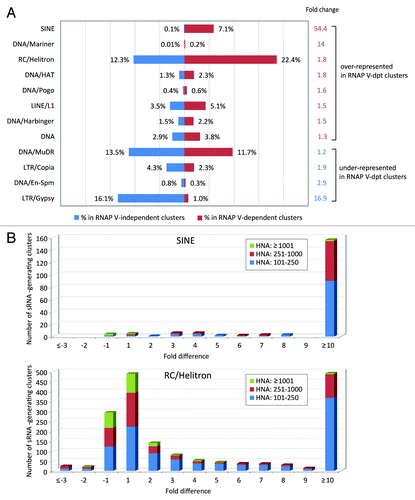
Figure 4. Repeat length is reversely correlated with the suppression of small RNA production in the nrpe mutant. (A) Plot of nrpe-dependent suppression of small RNA abundance vs. the length of repetitive sequences. The HNA was summed for the abundance of small RNAs mapped to the length of each annotated repetitive sequence, i.e., small RNA abundance for each repeat. Y-axis shows the Log10 scale of fold difference of sum of HNA between wild-type and nrpe libraries. The best-fit trendline from the power regression is showed. The section within 5 kb repeat length is further expanded and shown in (B). (B) Degree of nrpe-dependent suppression. The width of boxes is proportional to the square root of the numbers of repeats. (C) Numbers of RNAP V-dependent, small RNA-generating repeats (dark red) vs. the total number of small RNA-generating repeats (light red) in different repeat classes are shown along with the percentage (the former vs. the latter). (D) Comparison of numbers of RNAP V-dependent, small RNA-generating repeats (red) and the total number of small RNA-generating repeats (light red) across the size of repeat in selected repeat families. (E) Length profiles of repeat-annotated small RNA-generating elements are plotted with fixed-width box. White boxes represent data from small RNA-generating elements, and red boxes represent data from RNAP V-dependent, small RNA-generating elements based on the criteria described in the main text. Median values for both data sets are shown. Repeat length between 250 bp to 300 bp is highlighted in blue. For box plots in (B) and (E), the box indicates the fold changes in the 25th to 75th percentile with the center bar indicating the median. The dashed lines indicate the range of values in the lower or upper quartiles, terminating at the minimum or maximum lengths.
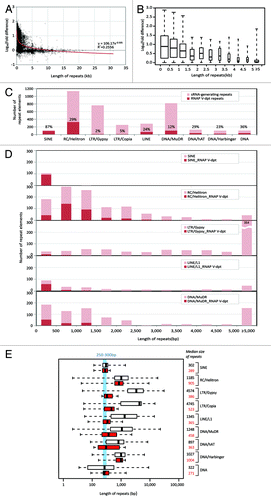
Figure 5. Assessment of the size of RNAP V-dependent regions. (A) Plot showing the degree of nrpe-dependent suppression on small RNA abundance across the width of RNAP V- dependent regions. One hundred of 500 bp, highly RNAP V-dependent clusters are selected (the average sum of HNA in nrpe libraries is less than 5 and HNA in Col is greater than 250 RP5M). Each selected cluster is joined together with its 500 bp upstream and 500bp downstream region for a 1,500 bp region. The degree of nrpe-dependent small RNA suppression is presented as the rounded fold-difference between Col vs. nrpe libraries in a window of 100 bp, sliding every 20 bp across the entire 1,500 bp region (gray background lines). For each of the RNAP V-dependent region, Gaussian distribution is used to fit the gray line of fold difference and shown as the red curve in the foreground. (B) The average curve was calculated (in red) from the data in (A) to represent the average size of a RNAP V-dependent region. The average width of a RNAP V-dependent locus was computed from the individual height and widths of each fitted curve in (A), independent of the exact position within the cluster (positions were ignored to focus on the shape of the curve for each cluster). The width of the average curve was calculated as 238 bp.
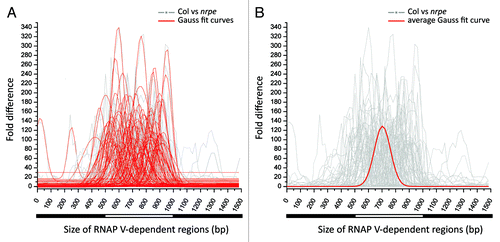
Figure 6. RdDM mutants show similar trends to the nrpe mutant of suppression of SINE-annotated siRNA-generating clusters. (A) Reduction of 24 nt small RNAs in RdDM mutant libraries. Shown for control [“Wt (T+S)”] and RdDM mutant libraries (drd1–1, drm3–1, rdm1–4 and dms4–1) are the 24 nt to 21 nt ratios of genome-matched small RNAs abundance (excluding structural RNAs). (B) Small RNA size profiles in control and RdDM mutant libraries. For each size class of small RNAs, the percentage of the small RNA abundance (excluding structural RNA) to the abundance of total genome-matched reads was calculated and normalized to the abundance of the 21 nt of wild-type libraries. (C) Number of clusters impacted in the RdDM mutants by the fold differences of small RNA abundance between control and RdDM mutant libraries. For each cluster, the fold difference of hit-normalized small RNA abundance between the control and RdDM libraries are calculated and rounded. Total numbers of clusters were tallied by the fold differences and plotted as data in Y-axis for each RdDM mutant library. These data and in particular the category of ≥ 10 is analogous to . (D) Pairwise comparison of RNAP V and RdDM effector-dependent. Numbers of clusters were calculated based on different criteria in nrpe, drd1–1, dms3–1, rdm1–4, dms4–1 libraries. Number of small RNA-generating clusters (criteria A) and RdDM effector-dependent, small RNA-generating clusters (criteria B) are shown for each RdDM mutant library. Pairwise comparison of nrpe vs. RdDM libraries (criteria C) shows the number of clusters which small RNA abundance is both greatly reduced in nrpe and RdDM mutant such as drd1- 1, i.e., RNAP V- and DRD1-dependent small RNA-generating clusters. (E and F) Area-proportional Venn diagrams show the number of small RNA-generating clusters which are suppressed in nrpe, dms4–1, and dms3–1 (E) and drd1–1, dms3–1, and rdm1–4 (F, left). The number of each sector in the Venn diagram in (f) is also shown in a representative Venn diagram (F, right). (G) Classes of repetitive sequences represented in RdDM effector-dependent small RNA-generating clusters. RNAP V-dependent (RNAP V-dpt) and RNAP V-independent (RNAP V-indpt) clusters are defined as described in the legend. RdDM effector-dependent clusters were selected with criteria B as described above. Repetitive sequences were identified by the TAIR9 repeat annotation. Proportion of repeat-annotated clusters to total number of RdDM-dependent loci for each control-RdDM mutant pair is shown on the y-axis (percentage).
![Figure 6. RdDM mutants show similar trends to the nrpe mutant of suppression of SINE-annotated siRNA-generating clusters. (A) Reduction of 24 nt small RNAs in RdDM mutant libraries. Shown for control [“Wt (T+S)”] and RdDM mutant libraries (drd1–1, drm3–1, rdm1–4 and dms4–1) are the 24 nt to 21 nt ratios of genome-matched small RNAs abundance (excluding structural RNAs). (B) Small RNA size profiles in control and RdDM mutant libraries. For each size class of small RNAs, the percentage of the small RNA abundance (excluding structural RNA) to the abundance of total genome-matched reads was calculated and normalized to the abundance of the 21 nt of wild-type libraries. (C) Number of clusters impacted in the RdDM mutants by the fold differences of small RNA abundance between control and RdDM mutant libraries. For each cluster, the fold difference of hit-normalized small RNA abundance between the control and RdDM libraries are calculated and rounded. Total numbers of clusters were tallied by the fold differences and plotted as data in Y-axis for each RdDM mutant library. These data and in particular the category of ≥ 10 is analogous to Figure 1C. (D) Pairwise comparison of RNAP V and RdDM effector-dependent. Numbers of clusters were calculated based on different criteria in nrpe, drd1–1, dms3–1, rdm1–4, dms4–1 libraries. Number of small RNA-generating clusters (criteria A) and RdDM effector-dependent, small RNA-generating clusters (criteria B) are shown for each RdDM mutant library. Pairwise comparison of nrpe vs. RdDM libraries (criteria C) shows the number of clusters which small RNA abundance is both greatly reduced in nrpe and RdDM mutant such as drd1- 1, i.e., RNAP V- and DRD1-dependent small RNA-generating clusters. (E and F) Area-proportional Venn diagrams show the number of small RNA-generating clusters which are suppressed in nrpe, dms4–1, and dms3–1 (E) and drd1–1, dms3–1, and rdm1–4 (F, left). The number of each sector in the Venn diagram in (f) is also shown in a representative Venn diagram (F, right). (G) Classes of repetitive sequences represented in RdDM effector-dependent small RNA-generating clusters. RNAP V-dependent (RNAP V-dpt) and RNAP V-independent (RNAP V-indpt) clusters are defined as described in the Figure 1 legend. RdDM effector-dependent clusters were selected with criteria B as described above. Repetitive sequences were identified by the TAIR9 repeat annotation. Proportion of repeat-annotated clusters to total number of RdDM-dependent loci for each control-RdDM mutant pair is shown on the y-axis (percentage).](/cms/asset/1cde4c60-4fba-4e57-8b6e-ed18affe03de/kepi_a_10920290_f0006.gif)