Figures & data
Figure 1. Treatment of NSCLC cells with honokiol reduces the levels of HDAC activity while increasing HAT activity. (A) Comparative analysis of basal levels of HDAC and HAT activity in four different NSCLC cell lines and non-neoplastic BEAS-2B cells using colorimetric assay kits. (B) A549 and H1299 cells were treated with various concentrations of honokiol (0, 20, 40 and 60 µM) or TSA (100 nm) for 24 or 72 h. Total HDAC activity was determined in nuclear extracts of the cells. Cells treated with TSA, an inhibitor of HDACs, served as a positive control. (C) Treatment of A549 and H1299 cells with honokiol for 72 h enhanced HAT activity in a dose-dependent manner. Data are expressed in terms of percent of control as the mean ± SD of 4 replicates. Significant difference vs. non-honokiol-treated control, ¶p < 0.001, †p < 0.01. (D) Treatment of cells with honokiol for 72 h reduces the expression levels of class l HDACs proteins. After treatment for 72 h, cells were harvested, nuclear extracts were prepared and subjected to western blot analysis. Histone H3 was used as a loading control. Representative blots are shown. The relative intensity (arbitrary) of each band after normalization for histone H3 is shown under each blot as the fold change compared with non-honokiol-treated control, which was assigned an arbitrary unit 1.0 in each case.
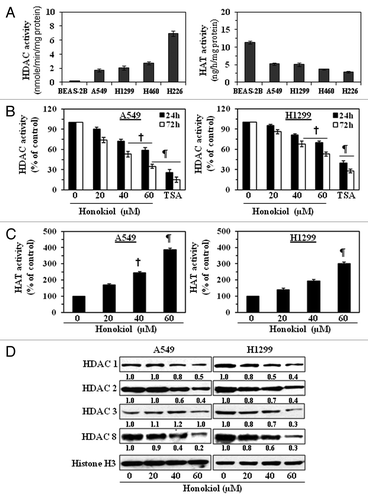
Figure 2. Effect of honokiol on acetylated histones and cell viability in NSCLC cells. (A) Treatment of NSCLC cells with MG132, a proteasome inhibiter, inhibits the effect of honokiol on HDAC proteins. A549 and H1299 cells were treated with honokiol (60 μM) with and without the treatment of MG132 for 72 h, then cells were harvested and nuclear lysates were subjected to western blot analysis. (B) Treatment of NSCLC cells with honokiol for 72 h enhances the levels of acetylated histone H3 and histone H4, as determined by western blot analysis. Equal loading of samples was verified by re-probing the membrane with anti-histone H3 antibody. The relative intensity of each band after normalization for the levels of histone H3 is shown under each blot. (C) Treatment of NSCLC cells with honokiol inhibits cell viability in a dose-dependent manner, but this effect of honokiol is not seen in BEAS-2B normal human bronchial epithelial cells. NSCLC cells and BEAS-2B cells were treated with various concentrations of honokiol for 48 h and cell viability determined using an MTT assay. (D) Honokiol (60 µM) significantly inhibited cell viability of NSCLC cells in a time-dependent manner, whereas a significant growth inhibitory effect was not observed in BEAS-2B cells. The cell viability data are expressed in terms of percent of control (non-honokiol treatment) cells as the mean ± SD of 5 replicates. Significant difference vs. control, ¶ p < 0.001 †p < 0.05 (left panel). Treatment of A549 and H1299 cells with MG-132 (10 μM), a proteasome inhibitor, reduced the cytotoxicity of honokiol (40 μM) in these lung cancer cells (right panel). Significant reduced cytotoxicity or increased cell viability vs. honokiol alone, †p < 0.05.
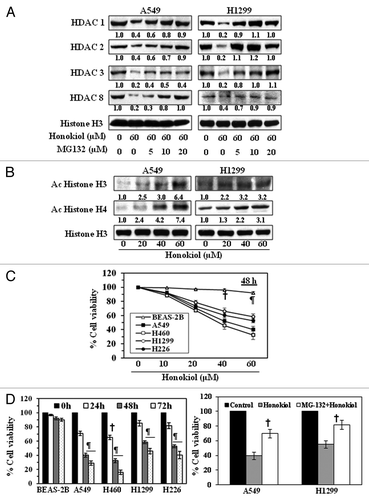
Figure 3. Effect of valproic acid, an inhibitor of HDACs, on the class I HDAC levels in, and the growth of, NSCLC cells. (A) Treatment of A549 and H1299 cells with valproic acid for 72 h inhibited the expression levels of class I HDACs. Histone H3 was used as a loading control. The relative intensity of each band after normalization for histone H3 is shown under each blot, and it is in terms of fold-change. (B) A549 and H1299 cells were treated with various concentrations of Valproic acid (0, 10 and 20 mM) for 48 and 72 h, and cell viability was determined using an MTT assay. Data on cell viability are presented in terms of percent of control (non-valproic acid-treated) as the mean ± SD of 4–5 replicates. (C) Treatment of NSCLC cells with valproic acid induces cell death. For cell death assay, 5 × 104 cells were plated in six-well culture plates and treated with or without valproic acid for 48 or 72 h. Cell death was determined using a trypan blue exclusion assay. Data are presented as the percent cell death as the mean ± SD from three separate experiments. Significant difference vs. non-valproic acid treated controls, ¶p < 0.001, †p < 0.05.
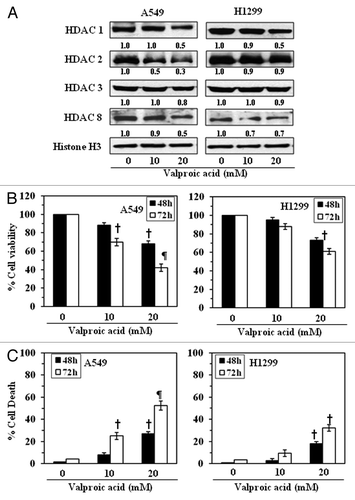
Figure 4. Effect of honokiol on cell cycle progression and apoptosis of A549 and H1299 cells. A549 (A) and H1299 (B) cells were treated with either vehicle or honokiol (0, 20, 40 and 60 µM) for 72 h. Cells were harvested, cellular DNA was stained with propidium iodide and flow cytometric analysis performed to analyze the cell cycle distribution. (C) Treatment of cells with honokiol for 72 h inhibits the levels of cyclins and CDKs associated with the G1 phase of the cell cycle in a dose-dependent manner, as analyzed by western blotting. The relative density of each band after normalization for β-actin is shown under each blot.
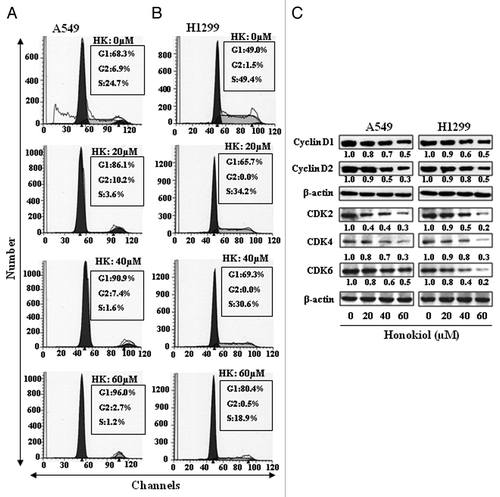
Figure 5. Administration of honokiol by gavage inhibits the growth of A549 or H1299 tumor xenografts in athymic nude mice. Mice were inoculated subcutaneously with 2 × 106 cells (A549 or H1299) on the right flank. Twenty-four hours later, mice were treated with either PBS (100 µL) or honokiol (100 mg/kg body weight/100 µL of PBS) by gavage twice per week for a total of eight weeks. (A) The body weight of the mice was monitored weekly. (B) Tumor volumes were recorded on a weekly basis to determine the chemotherapeutic effect of honokiol on the growth of tumor xenografts, and data are presented as tumor volume ± SD/mouse (mm3) in each group. (C) Tumors were harvested at the termination of the experiment, and the wet weight of the tumor/mouse in grams was measured and is reported as a mean ± SD. Statistical significance vs. non-honokiol-fed control group, ¶p < 0.01.
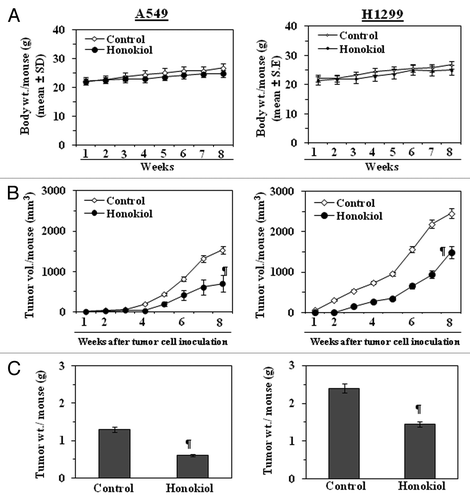
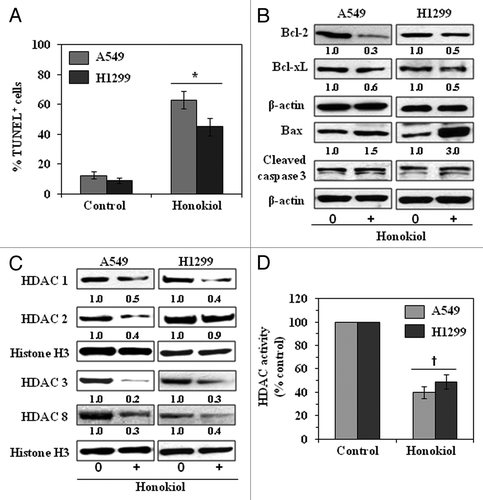
Figure 6. Administration of honokiol by oral gavage induces apoptosis and lowers the levels of class I HDACs in A549 or H1299 tumor xenografts. At the termination of the experiment described in , tumors were harvested and subjected to immunostaining and western blot analysis. (A) Percent of TUNEL-positive cells in sections of tumor xenograft tissues from honokiol-treated and honokiol-untreated mice. Significant increase vs. non-honokiol-treated controls, *p < 0.005. (B) Honokiol treatment inhibits the expression of anti-apoptotic proteins while increasing the expression of pro-apoptotic protein Bax and enhancing the activation of caspase-3 as determined by western blotting of the A549 and H1299 xenograft tissues. (C) Honokiol treatment inhibits the levels of class I HDACs as determined by western blotting of A549 and H1299 tumor xenograft tissues. Equal loading of samples was verified using the antibodies against histone H3 or β-actin. Relative intensity of each band compared with non-honokiol-treated control group is shown under each blot after normalization for β-actin or histone H3. (D) Total HDAC activity was determined in nuclear extracts of tumor samples using a colorimetric assay kit. Significant inhibition of HDAC activity vs. non-honokiol-treated controls, †p < 0.01.