Figures & data
Figure 1. Schematic representation of G9a domain structure. Human and mouse G9a isoforms are shown with their respective NCBI accession ID (left). The domain structure was constructed using DOG 1.0 software. The Cysteine (Cys) rich region, ankyrin repeats (ANK) and the catalytic SET domain with flanking Pre SET and post SET regions are shown. The site for methylation (Me),nuclear localization signal (NLS) and the glutamic acid (E) rich region are denoted. Numbers indicate amino acid residues.
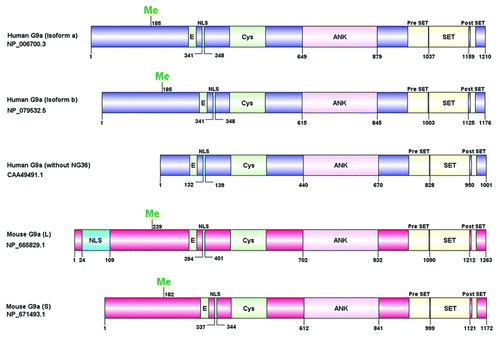
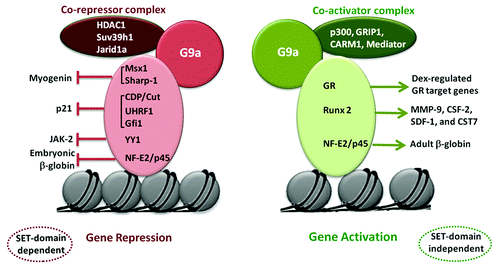
Figure 2. Transcriptional repression and activation by G9a. G9a recruitment by transcription factors and association with distinct co-factor complexes leads to opposing outcomes on gene expression. Transcriptional repression of genes such as myogenin, p21, JAK2 and embryonic β-globin is dependent on G9a methyltransferase activity (SET domain). On the other hand, G9a recruitment by GR, Runx2 and NF-E2/p45 leads to activation of target genes in a SET-domain independent manner. This occurs through association of G9a with co-activators such as p300 and CARM1, as well as the Mediator complex.