Figures & data
Figure 1. Schematic illustration of miRNAs function in neuronal fate determination during embryonic and adult neurogenesis. Neuroepithelial cells give origin to radial glia, which in turn undergo self-renew via symmetric (not shown) and asymmetric cell divisions (black arrows) in order to amplify the pool of NSCs during embryonic neurogenesis. A variety of neurons are originated from NSCs and intermediate progenitors via sequential fate restrictions regulated by crosstalk between miRNAs, signaling pathways and the epigenetic machinery as indicated by squares. Some radial glia cells are converted into astrocytic stem cells (B cell). B cells give origin to neuronal progenitors (C cell), which are the direct progeny of committed neuroblast (A cell) that differentiate into mature neuron. miRNAs associated with regulation of NSCs maintenance and neuronal commitment are shown in circles. Colored arrows indicate whether the cellular response is increased (↑) or decreased (↓).
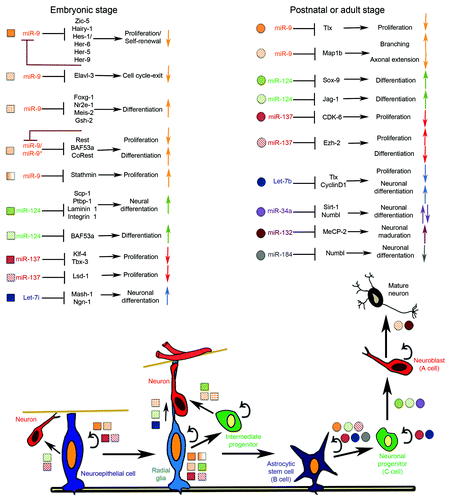
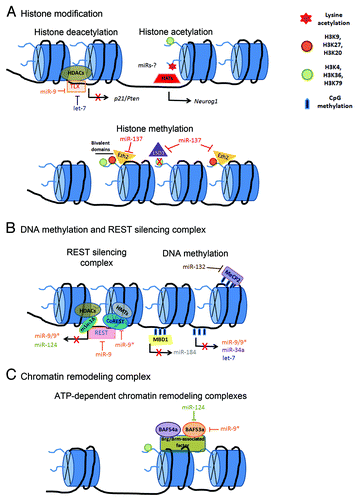
Figure 2. Molecular mechanisms of the epigenetic-miRNAs regulatory network associated with chromatin remodeling during neurogenesis. (A) Histone modifications are mediated by acetylation, deacetylation and methylation. HATs relax chromatin structure and facilitate transcription, while HDACs increase chromatin condensation and repress transcription. miR-9 and let-7 regulate the expression of the transcription factor TLX that directly recruits HDACs in order to mediate p21 and Pten gene repression in NSCs. miR-137 has emerged as an important regulator of histone methylation regulatory pathway by targeting the demethylase, LSD1, and the H3K27 methyltransferase, Ezh2. miR-137-Ezh2 network might be associated in balancing gene-specific bivalent domains in NSCs.Citation2 (B) DNA methylation is associated with transcriptional repression and negative regulation of several brain-enriched miRNAs. The transcriptional repressor MBD protein, MeCP2, binds to methylated genes in order to silence genes. MeCP2 has been identified as a target of miR-132 in neurons. The transcription factor REST regulates the expression of brain-enriched miR-9/9* and miR-124. miR-9/9* act as a negative feedback regulatory loop on REST silencing complex. (C) ATP-dependent chromatin remodeling complex alter the chromatin structure in order to regulate NSC fate determination. BAF53a expression levels are regulated by miR-124 and miR-9* during the transition between NSCs proliferation to differentiated post-mitotic neuron.Citation122