Figures & data
Figure 1. CagA affects multiple signaling pathways within gastric epithelial cells. CagA is translocated into host epithelial cells by the cag type IV secretion system, and undergoes tyrosine phosphorylation by Src and Abl kinases. Phospho-CagA interacts with and activates several host cell proteins, including a host cellular phosphatase (SHP-2), leading to increased motility and proliferation. Non-phosphorylated CagA directly binds PAR1b, a central regulator of cell polarity, and inhibits its kinase activity, an interaction that promotes loss of cell polarity. CagA in an unphosphorylated form activates β-catenin, leading to transcriptional upregulation of genes implicated in cancer. The CagA protein of certain H. pylori strains also can induce NFκB activation. Thus, the entry of CagA into host cells activates multiple signaling pathways that may increase the risk for malignant transformation.
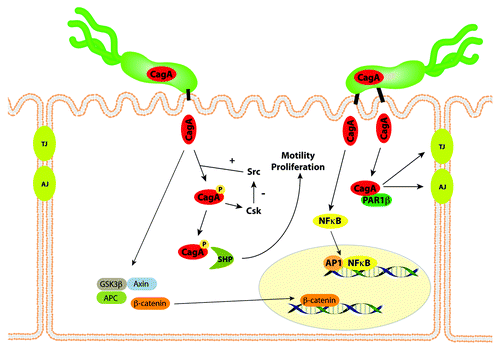
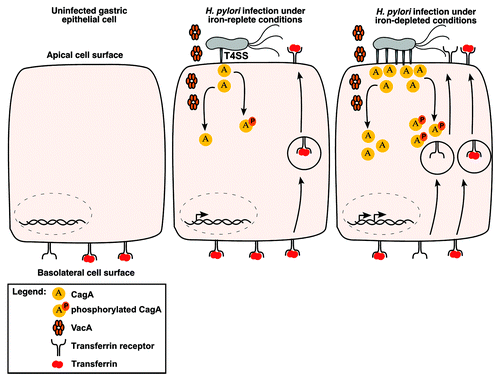
Figure 2. Effect of low-iron conditions on interactions between H. pylori and host cells. CagA and VacA each induce mislocalization of transferrin receptors from the basolateral cell surface to the apical surface where H. pylori are localized. Under iron-deplete conditions, CagA production is increased, the formation of pili associated with the cag type IV secretion system is increased, and translocation of CagA into host cells occurs at increased levels. This potentially leads to a more robust mislocalization of transferrin receptors to the apical membrane.