Figures & data
Table 1. Number of subjects included in the LT-ATP cohort at Year 17 with reasons for elimination
Table 2. Percentage of subjects seropositive for anti-HAV antibodies at each yearly follow-up time point up to 17 y (Long-term Total cohort and LT ATP cohort)
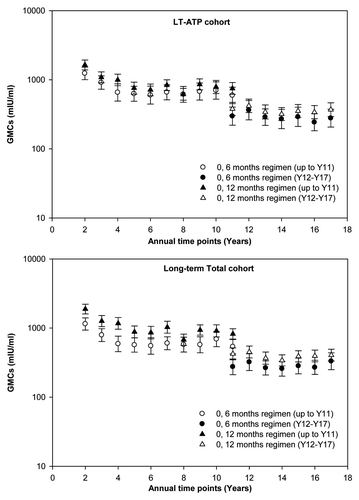
Figure 1. Evolution of anti-HAV antibody geometric mean concentration across the yearly follow-up time points. *Serum samples up to Year 11 were tested with ELISA kits, while serum samples from Years 12 to 17 were tested with EIA kits. Blood samples at Year 11 were re-tested with the new assay kit and results were compared with the previous assay kit. Anti-HAV seropositivity cut-offs: Up to Year 11: ≥ 20 mIU/ml; Year 12 to Year 17: ≥ 15 mIU/ml