Figures & data
Table 1. Composition of study vaccines
Table 2. Demographic characteristics of the total vaccinated cohort
Figure 1. Comparison between groups in the immune response to poliovirus types 1, 2 and 3 (ATP immunogenicity cohort): (A) difference in seroprotection rates between the control group and each DTPa-HBV-IPV/Hib formulation (Formulations 1–3); (B) GMT ratios (post divided by pre-vaccination titers) for anti-poliovirus types 1, 2 and 3. (A) The upper limits of the standardized asymptotic 95% CI on the group difference in the percentage of subjects with anti-poliovirus types 1, 2 and 3 titers ≥ 8 are ≤ 10 (predefined criteria for non-inferiority indicated by bold horizontal line); (B) The lower limits of the two-sided 95% CI on the geometric mean of the individual ratios (post- over pre-vaccination titers) for anti-poliovirus types 1, 2 and 3 antibodies are ≥ 2 (predefined criteria for immunogenicity indicated by bold horizontal line). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.
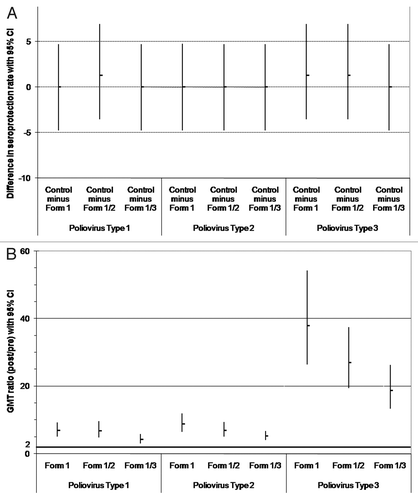
Table 3. Seroprotection/seropositivity rates and antibody GMCs before and one month post-booster (ATP cohort for immunogenicity)
Figure 2. Seroconversion rates for poliovirus types 1, 2 and 3 one month post-booster (ATP immunogenicity cohort). *Seroconversion was defined as post-booster antibody titer ≥ 1:8 in initially seronegative subjects, at least a 4-fold increase in post-booster titer in initially seropositive subjects, or a titer greater than the highest dilution tested [1:8192] in subjects with pre-booster antibody titers < 8192). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.
![Figure 2. Seroconversion rates for poliovirus types 1, 2 and 3 one month post-booster (ATP immunogenicity cohort). *Seroconversion was defined as post-booster antibody titer ≥ 1:8 in initially seronegative subjects, at least a 4-fold increase in post-booster titer in initially seropositive subjects, or a titer greater than the highest dilution tested [1:8192] in subjects with pre-booster antibody titers < 8192). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.](/cms/asset/f3542aa2-7ca4-41ce-8825-4aac9d4071a7/khvi_a_10918630_f0002.gif)
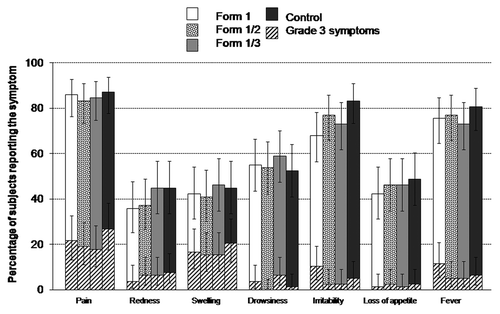
Figure 3. Percentage of subjects reporting solicited local and general symptoms during the 8-d post-vaccination follow-up period (total vaccinated cohort). Grade 3 was defined as: Cried when limb is moved/spontaneously painful (pain); injection site diameter > 20 mm (redness/swelling); axillary temperature > 39.0°C (fever); prevented normal daily activities (irritability/fussiness, drowsiness); not eating at all (loss of appetite). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib + IPV.