Figures & data
Figure 1. Phylogenetic trees based on neighbor-joining evaluation of E6 and E7 alignments (A and B, respectively). Asterisks indicate location of consensus sequences on each tree. (C) Schematic design of p6E6E7 and p11E6E7 constructs.
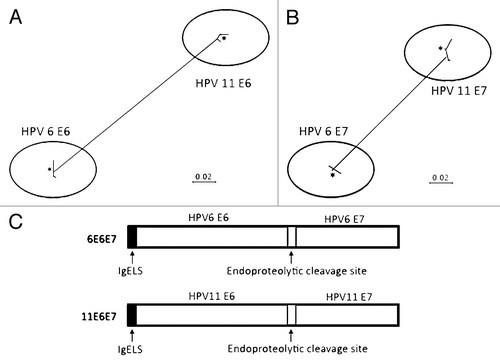
Figure 2. In vivo expression of p6E6E7 and p11E6E7. Gene products were isolated from lysed transfected 293-T cells, run through SDS-PAGE gel, and detected using autoradiography. Both HPV 6 and HPV 11 E6/E7 proteins are approximately 32kDa each (A). Human rhabdomyosarcoma (RD) cells were also transfected with p6E6E7 and p11E6E7 and later fixed after immunofluorescence staining. FITC fluorescence confirms expression of p6E6E7 and p11E6E7 (B). DAPI fluorescence confirms nuclei localization consequent of Hoechst staining.
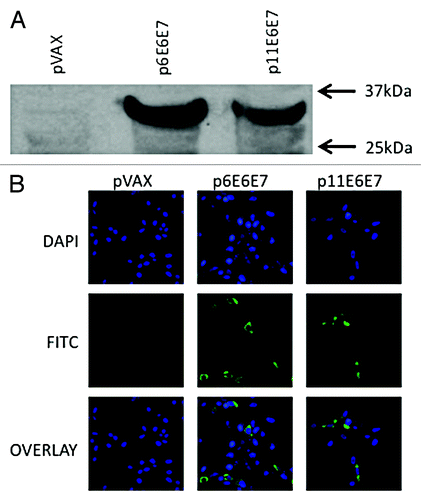
Figure 3. IFN-γ ELISpot assays show induction of robust cell-mediated responses by p6E6E7 (A) and p11E6E7 (B) in C57BL/6 mice. Assays were performed using splenocytes isolated from mice in each respective group (5 mice per group) after three biweekly immunizations. Each immunization consisted of 20μg per construct. Mice in combo group received both p6E6E7 and p11E6E7 at 20μg per construct, for a total of 40μg DNA per immunization. DNA was administered via IM injection, followed by electroporation (* denotes p < 0.05).
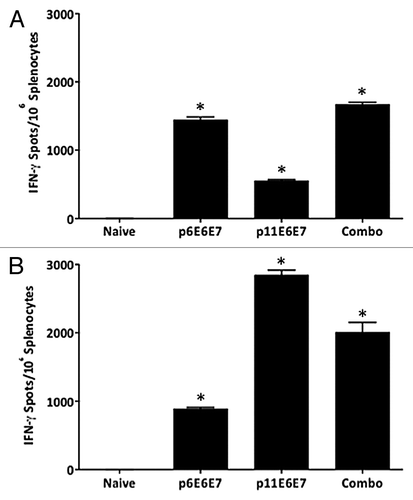
Figure 4. Additional IFN-γ ELISpot assays performed using individual peptides to characterize dominant epitopes. Splenocytes isolated from vaccinated mice and negative control were stimulated with overlapping peptides that span the entire HPV 6 E6/E7 fusion protein (A) or HPV 11 E6/E7 fusion protein (B).
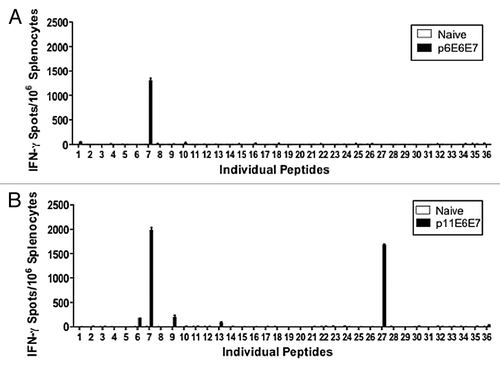
Figure 5. Schematic diagram of gating strategy of T cells response to the HPV6- and HPV11-specific peptides.
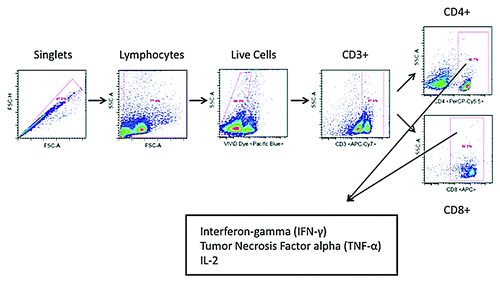
Figure 6. Cytokine production by HPV6 antigen-specific T-cells characterized by intracellular cytokine staining. Splenocytes isolated from mice vaccinated with p6E6E7 were stimulated with R10 growth medium, PMA, or consensus gene peptides for 4 h prior to surface marker and intracellular staining. Dot plots above show differences in background-subtracted percentages of either total CD4+ or CD8+ cells producing IFN-γ, IL-2, and TNF-α.
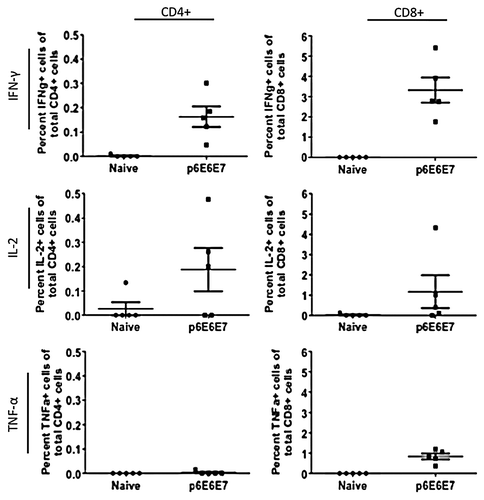
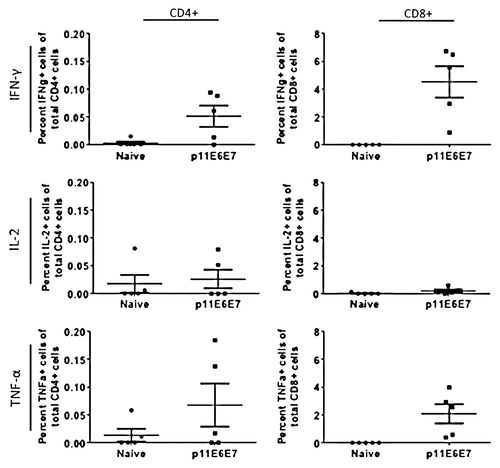
Figure 7. Cytokine production by HPV11 E6E7-specific T-cells quantified by intracellular cytokine staining. As with those cells used to measure HPV6 antigen-specific T-cell cytokine production, splenocytes from mice vaccinated with p11E6E7 were isolated and stimulated with R10 growth medium, PMA, or consensus gene peptides. The cells were subsequently processed for surface marker and intracellular staining. Dot plots above portray percentage of total Cd4+ or CD8+ cells producing IFN-γ, IL-2, and TNF-α. All values graphed have been background-subtracted.