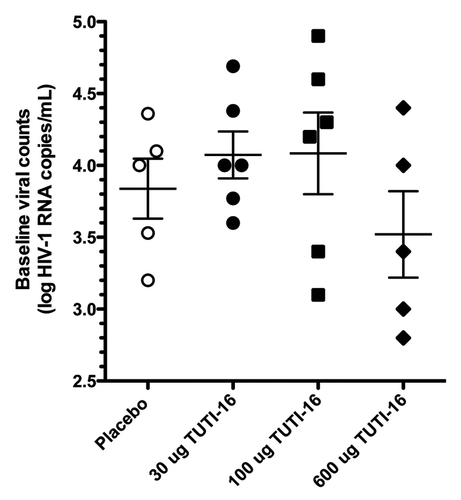
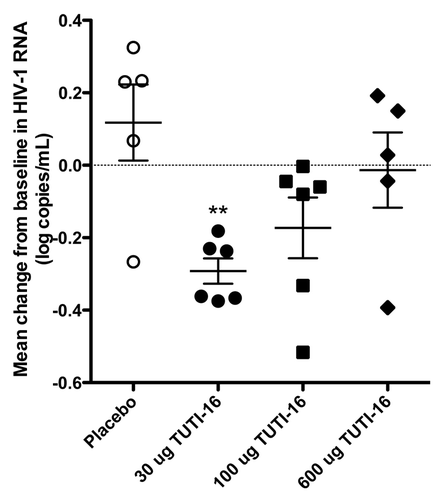
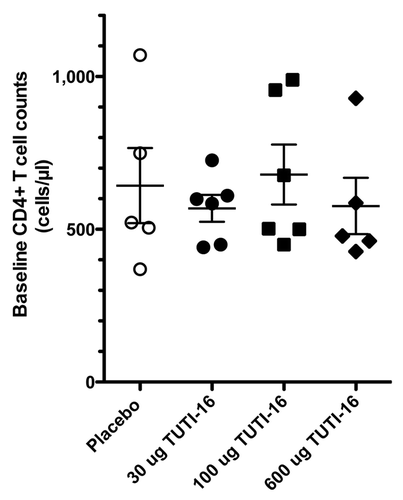
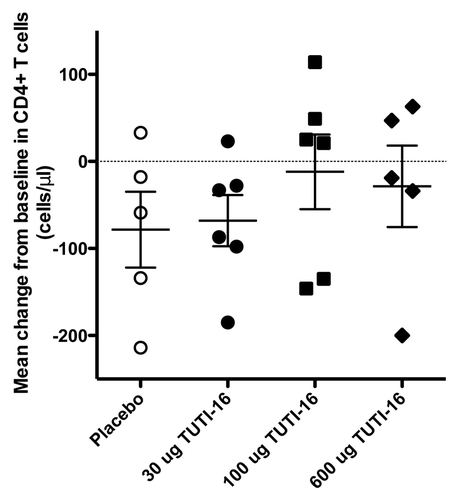
Figures & data
Table 1. Baseline demographics and injection related adverse reactions
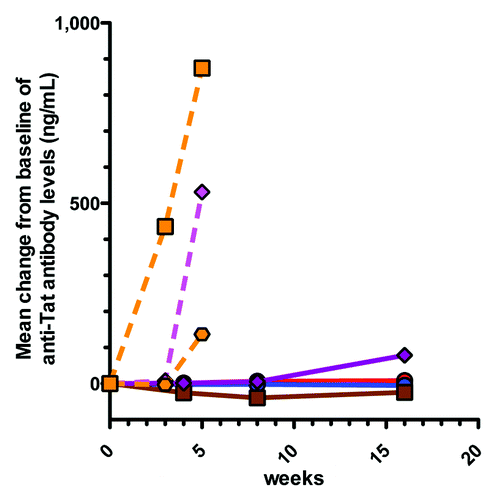
Figure 2. Mean changes from baseline of anti-Tat antibody concentrations following TUTI-16 immunization. Treatment naïve HIV subjects (solid lines): placebo red circle, 30 μg TUTI-16 blue circle, 100 μg TUTI-16 brown square, 600 μg TUTI-16 plum diamond. Healthy HIV seronegative subjects (dashed lines): 200 μg TUTI-16 orange hexagonal, 1.0 mg TUTI-16 orange square; ART controlled HIV subjects: 200 μg TUTI-16 magenta diamond. Treatment naïve HIV randomized study immunizations at 0, 4 and 12 weeks; open label immunizations at 0 and 3 weeks.