Figures & data
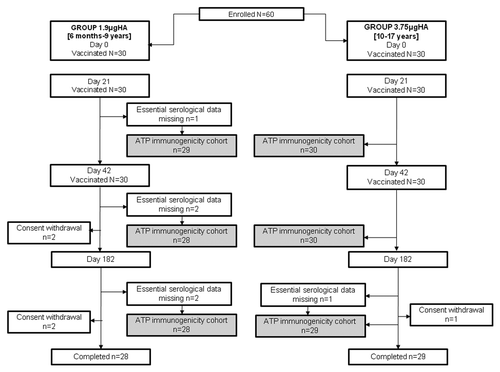
Figure 1. Study design diagram. Total vaccinated cohort (TVC): all subjects with at least one documented vaccine dose with available immunogenicity results. According-to-protocol (ATP) cohort for immunogenicity: all evaluable subjects (i.e., those meeting all eligibility criteria, with no elimination criteria during the relevant analysis interval), who received two vaccine doses and for whom assay results were available at Day 42 and Day 182. Group 1.9µgHA: Subjects aged 6 mo-9 y received two doses of 1.9µg HA+AS03B (5.93mg α-tocopherol) vaccine, 21 d apart. Group 3.75µgHA: Subjects aged 10–17 y received two doses of 3.75µg HA+AS03A (11.86mg α-tocopherol) vaccine, 21 d apart.