Figures & data
Figure 1. (A) SDS-PAGE under reducing conditions containing 2 µg of Na-GST-1 in 50 mM sodium acetate pH 6.0 buffer. Lane A represents reference protein stored at < -50°C and Lanes B-E are replicate samples of the recombinant protein stored at 2–8°C. (B) SDS-PAGE under non-reducing conditions of Na-GST-1 samples. Lanes A and B correspond to supernatant from two protein samples where particulates were observed after freeze thaw. Lanes C and D represent protein samples from lanes A and B after they were centrifuged to isolate particulates and resuspended in sample buffer. (C-D) SE-HPLC of two recombinant Na-GST-1 protein samples. Panel C represents recombinant protein stored at freezing temperature (below -50°C). Panel D represents recombinant protein stored at 2–8°C for 3 mo with a decrease in main peak area, purity and detection of lower molecular weight breakdown products.
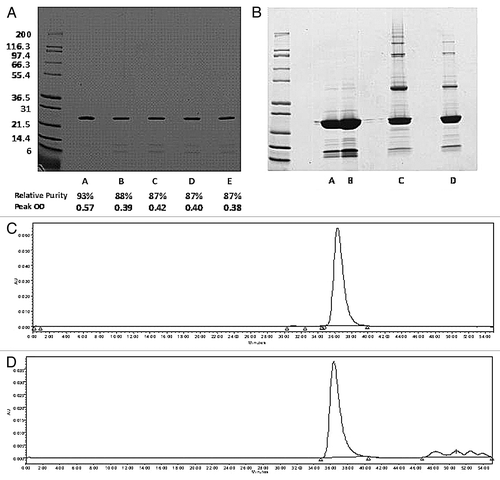
Figure 2. Ezymatic activity of 40 µg Na-GST-1 protein measured by fluorescence assay and reported in relative fluorescence units (RFU) under different treatment conditions: Control = untreated (frozen protein and thawed for use); heat treated at two temperatures (55°C and 100°C) and under reducing conditions with DTT). Results shown are average of duplicate measurements.
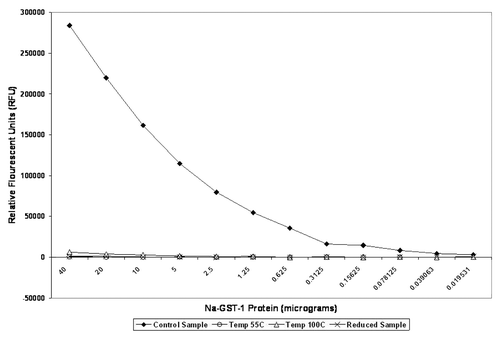
Figure 3. (A) The effect of ph on the secondary structure of Na-GST-1 at 10◦C. Each data point represents individual measurements of CD spectra from a single representative sample. (B) Ellipticity at 222 nm as a function of temperature was measured over the pH range of 3–8. Duplicate measurements were taken and the average value displayed. (C) Tryptophan emission fluorescence peak position of Na-GST-1 as a function of pH and temperature. Na-GST-1 at pH 3–8 was heated from 10 – 87.5°C, and the fluorescence emission maximum was determined after excitation at 295 nm. (D) Tryptophan emission fluorescence intensity of Na-GST-1 as a function of pH and temperature. Na-GST-1 at pH 3–8 was heated from 10 – 87.5°C. Error bars are from duplicate measurements.
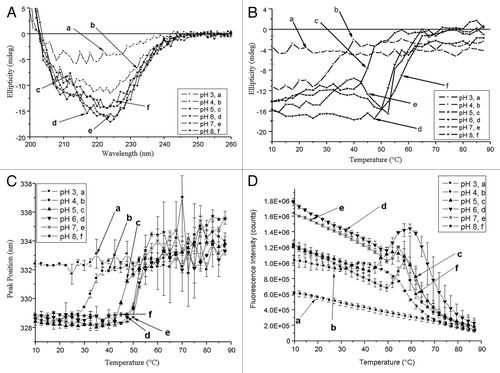
Figure 4. (A) Static light scattering as a function of pH and temperature. Na-GST-1 at pH 3–8 was heated from 10 – 87.5°C and the scattering intensity was monitored at 295 nm. Error bars are from duplicate measurements. (B) Binding of ANS to Na-GST-1 as a function of pH and temperature. A 20:1 ANS:protein molar ratio was excited at 372 nm and the fluorescence intensity at 484 was monitored as a function of temperature. Error bars are from duplicate measurements.
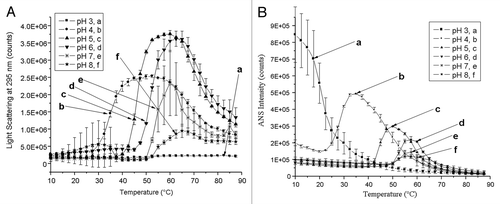
Figure 5. Empirical phase diagram (EPD) derived from biophysical characterization of Na-GST-1. The EPD is prepared from temperature-dependent intrinsic tryptophan fluorescence peak position, peak intensity, extrinsic ANS fluorescent peak intensity, circular dichroism at 222 nm, and static light scattering (see text).
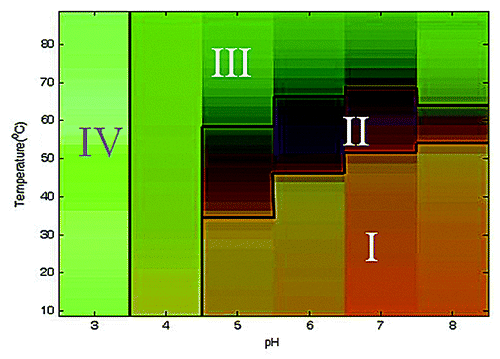
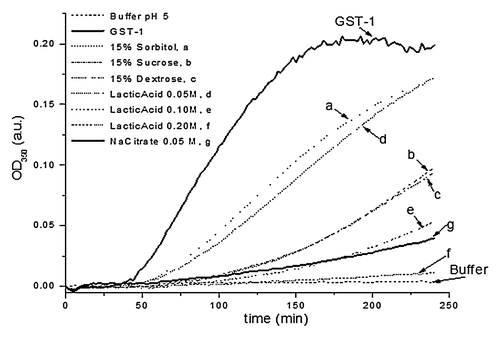
Figure 6. Excipient screening of Na-GST-1 using OD350 measurements. The change in optical density of Na-GST-1 is displayed as a function of time. Selected results are shown as an example. Experiments were conducted at pH 5 and 50°C.