Figures & data
Table 1. Evaluation of primary non-inferiority hypotheses and exploratory analyses: Coad group vs. ACWY-TT and MenPS groups (ATP Influenza cohort for immunogenicity)
Figure 1. rSBA GMTs in each group one month after vaccination (ATP Influenza cohort for immunogenicity). *statistically significant difference between the indicated group and the Coad group: differences between groups were done on GMT values adjusted for pre-vaccination measurements and age strata, exploratory analysis, whereas the GMTs displayed are unadjusted.
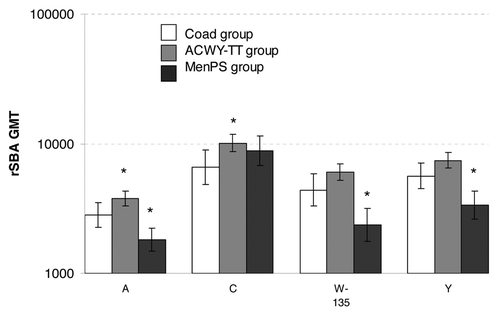
Figure 2. Reverse cumulative curves showing rSBA titers for N. meningitidis serogroups A, C, W-135 and Y. ACWY+F, Coad group; ACWY_F, ACWY-TT group; MenPS_F, MenPS group
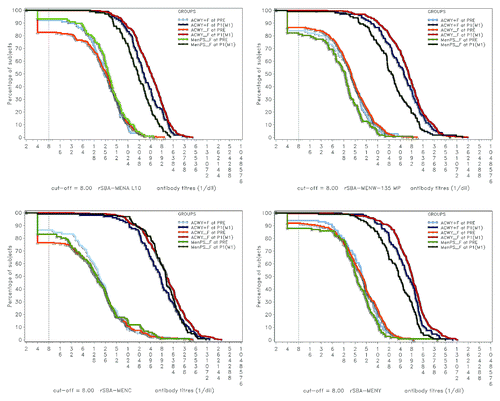
Table 2. Influenza humoral immune responses one month after vaccination (ATP Influenza cohort for immunogenicity)
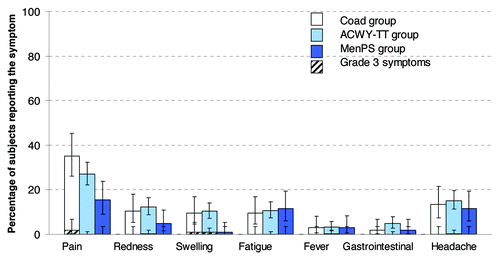
Figure 3. Percentage of subjects reporting solicited local and general symptoms during the 4-day post-vaccination period (total vaccinated Influenza cohort). Note: For the Co-ad group, local symptoms refer to the percentage of subjects with at least one local symptom at either injection site. Gastrointestinal symptoms included nausea, vomiting, diarrhea and/or abdominal pain. Grade 3: Redness and swelling > 50mm, fever > 39.5°C, Preventing normal everyday activity for all other symptoms.