Figures & data
Figure 1. Participant flow. ACWY_T, toddlers vaccinated with MenACWY-TT at 12–14 mo of age; MenCCRM, toddlers vaccinated with MenC-CRM197 at 12–14 mo of age; ACWY_C, children vaccinated with MenACWY-TT at 3–5 y of age; MenPS, children vaccinated with MenPS at 3–5 y of age; ATP, according to protocol; TVC, total vaccinated cohort; N, number of participants. 1Several formulations were evaluated in the primary study, but participants who received the selected formulation for further development or control vaccine were eligible to participate in the persistence study. Participants who withdrew from the primary phase were described in the previous publication.Citation6
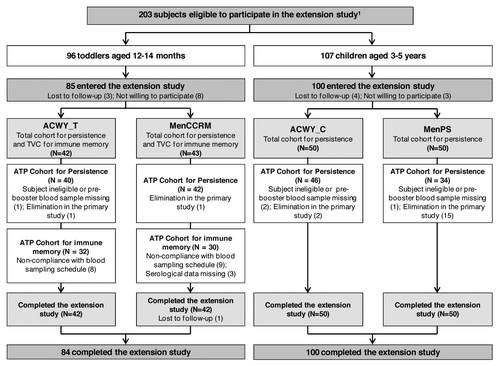
Table 1. Percentage of participants with rSBA titers above defined thresholds at pre-vaccination, at 1 mo and 15 mo after primary vaccination (ATP cohort for persistence) and at 1 mo after the polysaccharide challenge (ATP cohort for immune memory)
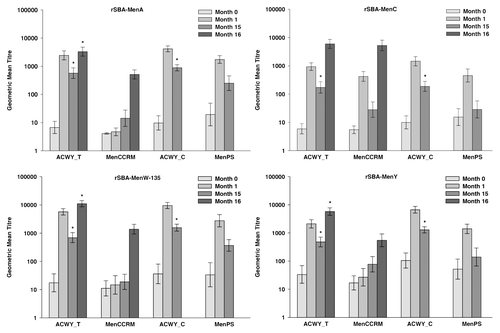
Figure 2. Geometric mean titers for rSBA at pre-vaccination, at 1 moCitation1 and 15 moCitation1 after primary vaccination (ATP cohort for persistence) and at 1 mo after the polysaccharide challenge (ATP cohort for immune memory). Notes: ATP, according to protocol; ACWY_T, toddlers primed with MenACWY-TT at 12–14 mo of age; MenCCRM, toddlers primed with MenC-CRM197 at 12–14 mo of age; ACWY_C, children primed with MenACWY-TT at 3–5 y of age; MenPS, children primed with MenPS at 3–5 y of age; Errors bars, exact 95% confidence interval; Month 0, pre-primary vaccination; Month 1, 1 mo post-primary vaccination; Month 15, 15 mo post-primary vaccination; Month 16, one month post-polysaccharide challenge. 1These numbers are not the same as those presented in the previous publication at the same timepoints because the analyses for the present study were conducted on the ATP cohorts for persistence and immune memory (Month 15) and in the previous publication the analyses were performed on the ATP cohort for immunogenicity (Month 1). *Statistically significantly higher values compared with the control group (exploratory analysis)