Figures & data
Figure 1. Participants’ progression through the study. ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; ATP, according to protocol; TVC, total vaccinated cohort; N, number of children; *Reasons for non-eligibility: suboptimal responder re-vaccinated with a monovalent meningococcal serogroup C vaccine (n = 41); suboptimal responder who did not come to the booster vaccination visit (n = 2); consent withdrawal (n = 1); and acute leukemia (n = 1)
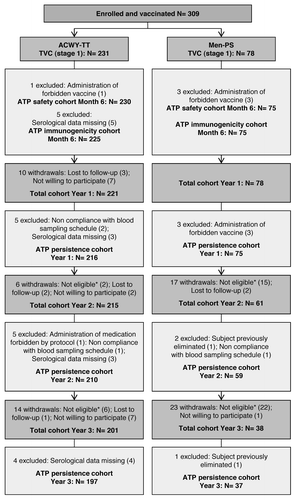
Table 1. Demographic characteristics of enrolled and vaccinated children (total vaccinated cohort)
Table 2. rSBA vaccine response rates at one month after administration of MenACWY-TT or MenACWY polysaccharide vaccine (ATP immunogenicity cohort)
Table 3. Percentage of children with rSBA titers equal to or above cut-off values, and rSBA GMTs before and one month after vaccination (ATP immunogenicity cohort), one year after vaccination (ATP persistence cohort Year 1), two years after vaccination (ATP persistence cohort Year 2) and three years after vaccination (ATP persistence cohort Year 3). ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; GMT, geometric mean antibody titer calculated on all children; N, number of children with available results; %, percentage of children with titer within the specified range; 95% CI, 95% confidence interval; Pre, pre-vaccination; Month 1, Year 1, Year 2, Year 3, one month, one year, two years and three years after vaccination. *Indicates higher value in the ACWY-TT group compared with the Men-PS group (exploratory analyses). Differences between groups in terms of GMTs are based on values adjusted for pre-vaccination measurements
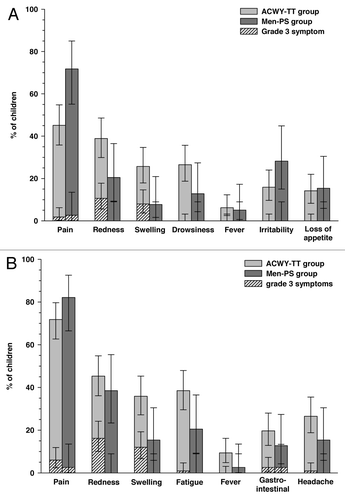
Figure 2. Incidence (with 95% CI) of solicited local and general symptoms occurring within 4 d after the first vaccination in children aged 2–5 y (A) or 6–10 y (B) (total vaccinated cohort). ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine. Error bars represent 95% confidence intervals