Figures & data
Table 1. Plant-produced VLP-based vaccines that have reached human clinical trial stage and FDA-approved plant-derived human pharmaceuticals
Figure 1. Production of West Nile virus enveloped VLP based on the prM/M and the E protein in N. benthamiana plants. Leaf tissue was infiltrated with Agrobacterium harboring the WNV prM-E construct. Leaf proteins were extracted 7 d post infiltration. PrM/M-E VLP was isolated by PEG precipitation and analyzed on 4–12% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were incubated with an anti-WNV E antibody (Lanes 1–3) or an anti-WNV M-E antibody (Lane 4). Lane1, Protein sample from buffer-infiltrated leaves; Lane 2, Purified WNV E protein as positive control; Lanes 3–4, Samples from prM-E construct-infiltrated plants. *: E protein; **: Unprocessed prM protein; ***: Processed M protein.
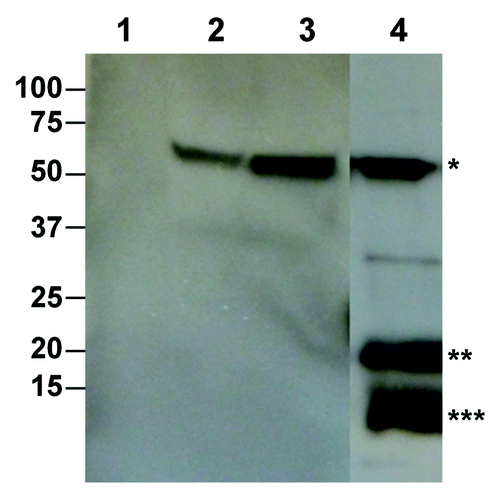
Table 2. Examples of plant-derived enveloped VLPs and cVLPs
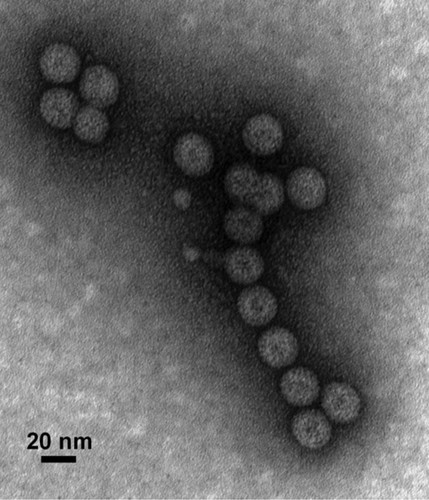
Figure 2. Production of chimeric HBcAg VLPs displaying West Nile virus Domain III of the envelope protein in plants. N. benthamiana leaves were infiltrated with Agrobacterium carrying the HBcAg and WNV DIII fusion construct. HBcAg-DIII cVLPs were purified from the infiltrated leaf tissue, stained with 0.2% aqueous uranyl acetate, and analyzed by transmission electron microscopy.