Figures & data
Table 1. Incidence of solicited local and general symptoms reported during the 4 d post-vaccination period following PHiD-CV booster dose (total vaccinated cohort)
Figure 2. Kinetics of serotype-specific OPA geometric mean titres (GMTs) for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, post-primary, pre-booster and post-booster vaccination with PHiD-CV (ATP immunogenicity cohort). ATP, according-to-protocol; OPA , opsonophagocytic activity; GMT, geometric mean titre.
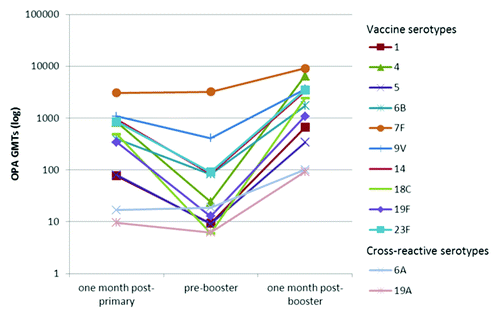
Table 2. Percentage of children with antibody concentrations ≥ 0.2 μg/ml (22F-inhibition ELISA) and OPA titers ≥ 8 for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, seropositivity rates and GMCs for anti-protein D antibodies pre- and 1 months post-booster dose (ATP immunogenicity cohort)
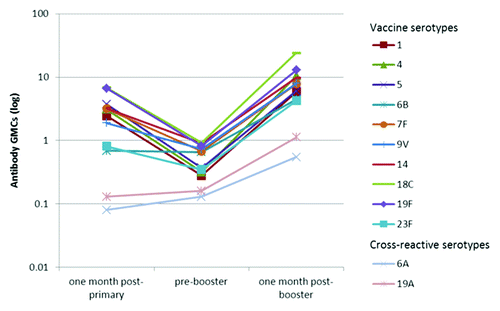
Figure 1. Kinetics of serotype-specific antibody geometric mean concentrations (GMCs, GSK’s 22F-inhibition ELISA) for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, post-primary, pre-booster and post-booster vaccination with PHiD-CV (ATP immunogenicity cohort). GMC, geometric mean concentration; ATP, according-to-protocol; GSK’s 22F-ELISA, GlaxoSmithKline’s 22F-inhibition enzymelinked immunosorbent assay. Note: Immunogenicity results related to one month post- primary were derived from the primary vaccination study NCT00678301 by re-calculation based on the ATP immunogenicity cohort of the booster study.