Figures & data
Figure 1. HVJ-Envelope exerts strong adjuvant activity by the induction of CTL/NK and repression of regulatory T cells via the activation of dendritic cells.
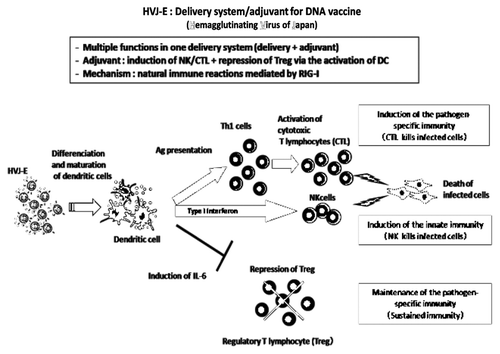
Table 1. ELISPOT assay for IFN-γ antigen-specific responses in the spleens of vaccinated mice following stimulation with HSP65 protein
Figure 2. Induction of CD8+ CTL specific for HSP65 protein and M. tuberculosis by vaccination with HVJ-Envelope/HSP65 DNA + IL-12 DNA. Spleen cells from the naïve, BCG-, and HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccinated mice were obtained eight weeks after the final vaccination. Cytotoxicity was assayed as release of radioactivity from 51Cr-labeled P815 target that had been transfected with HSP65 DNA using conventional 51Cr release assay as described in Materials and Methods.
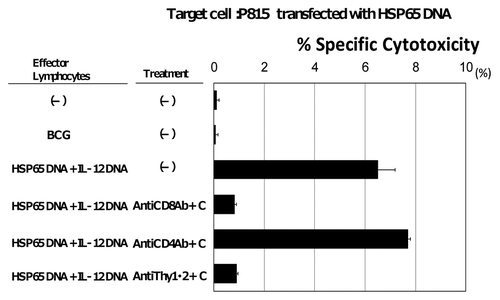
Figure 3. Induction of CD8 positive CTL against TB by HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine, and differentiation of CTL.
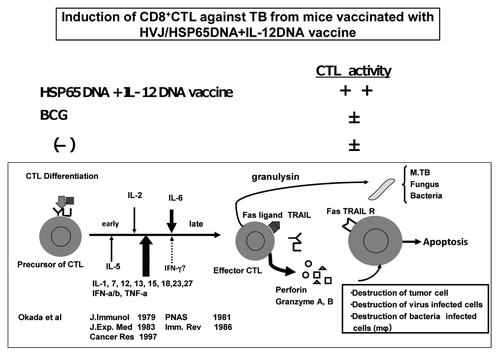
Table 2. The in vivo necessity CD8 positive T cells and CD4 positive T cells for prophylactic efficacy of the HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine
Figure 4. IFN-γ and IL-2 production efficacy of HSP65+IL-12/HVJ and BCG using prime-boost method against TB challenged cynomologus monkeys. Group of animals were vaccinated three times (every three weeks) with (1○) BCG Tokyo, (2○) HSP65 +IL-12/HVJ, (3○) HSP65+IL-12/HVJ = G1(■) BCG prime-HVJ/DNA boost group; (1○) HSP65+IL-12/HVJ, (2○) HSP65 +IL-12/HVJ, (3○)BCG = G2(□) HVJ/DNA prime-BCG boost group; (1○) HSP65+IL-12/HVJ, (2○) HSP65 +IL-12/HVJ, (3○) HSP65+IL-12/HVJ = G3(▩); (1) BCG, (2○)saline, (3○) saline = G4(▥) G4 group animals were vaccinated with BCG once.; (1○) saline, (2○)saline, (3○) saline = G5 (▤). (A) PBL from vaccinated monkeys on 56 d or 112 d after 1st immunization, were cultured for three days. IFN-γ activity on 56 d and IL-2 activity (B) on 112days were assessed by ELISA.
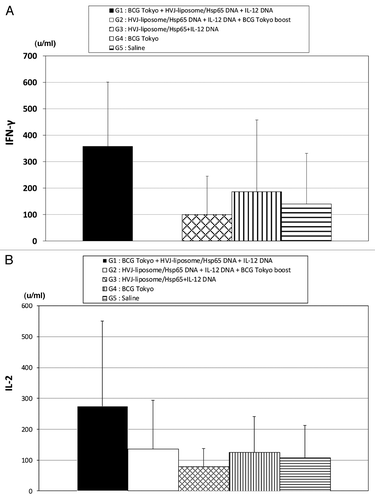
Table 3. IL-2 production from PBL in the cynomolgus monkeys
Table 4. In vitro and in vivo CTL induction by 15K granulysin
Figure 5. Differentiation of CTL by 15K granulysin and positive feedback loop of 15K granulysin in CTL induction. Precursor CTL are activated by IL-2 in the early stage of CTL induction of five day MLC. On the other hand, IL-6 acts, as a cytotoxic T cell differentiation factor, on the late stage of CTL induction as shown by Okada, et al. (J.I. 1988). 15K granulysin is produced from effector CTL, and induced the differentiation of CTL as a CTL differentiation factor. (Positive feedback loop by 15K granulysin.)
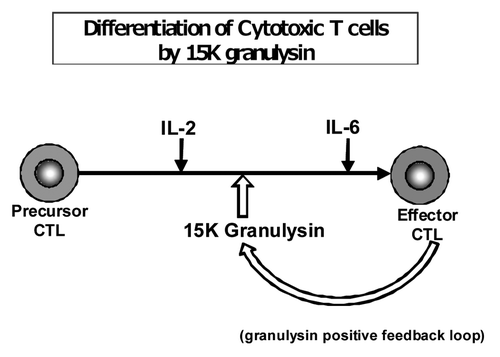
Figure 6. Induction of CTL differentiation by killer-specific secretory protein of 37Kd (Ksp37). Splenic T cell from C57BL/6 mice (H-2b) were obtained as described in (PNAS Okada et al. 1981), and cultured with uv-treated BALB/c (H-2d) spleen cells (Mitomycin C treated) in the presence of 100ng/ml rKsp37 and/or an anti-Ksp37 antibody for 5 d. CTL activity against P815 tumor cells (H-2d) was assessed by using 51Cr assay.
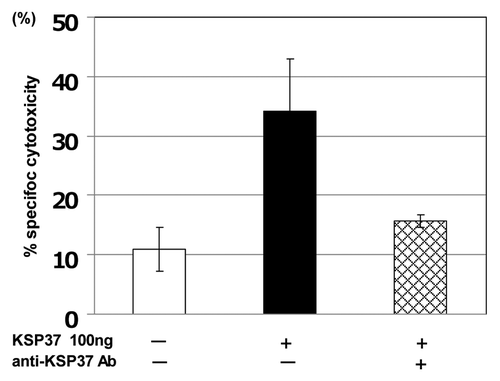
Figure 7. Augmentation of cytokines (IL-2, IFN-γ and IL-6) production by Ksp37 was also observed. (A) Augmentation of IL-2 production from T cells or spleen cells by Ksp37. An amount of 5 × 106 splenic T cells or spleen cells from C57BL/6 mice were cultured with 5 × 105 BALB/c spleen cells (Mitomycin-C treated) in the presence of rKsp37 for two days. IL-2, IFN-γ and IL-6 activities in the supernatants were assessed by ELISA. (B) Augmentation of IFN-γ production from spleen cells by Ksp37. (C) Augmentation of IL-6 production from spleen cells by Ksp37.
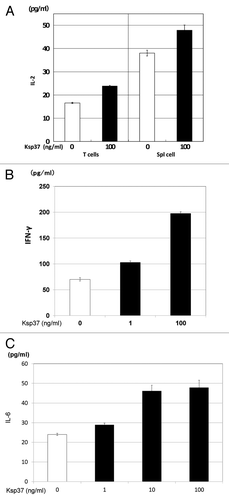
Figure 8. Augmentation of CTL differentiation in vivo by the treatment with Ksp37 DNA was demonstrated. C57BL/six mice were challenged with killed H37Ra antigen (1,000 µg/mouse) in vivo (i.p.) and then treated with Ksp37 DNA (100 µg/mouse, six times) i.m. Twenty-one days after challenge of killed TB antigen, and PEC (peritoneal exudate cells) from these mice were harvested. CTL activity againt TB antigen (HSP65 antigen) was assessed by using 51Cr EL-4 which had been transfected with HSP65 DNA.
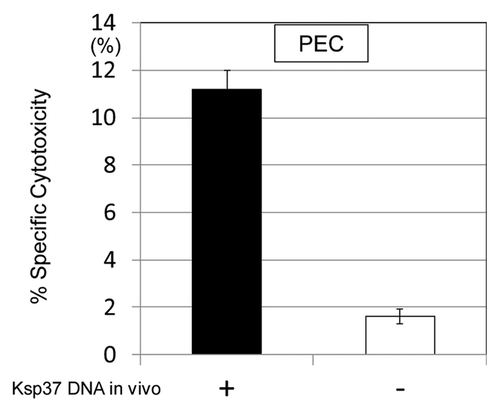
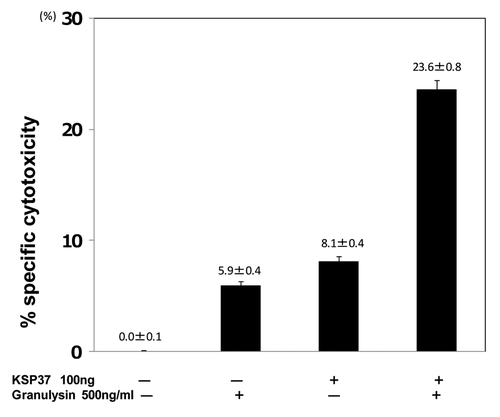
Figure 9. Synergistic efficacy of Ksp37 and granulysin on the in vitro CTL induction. Splenic T cells were cultured with uv-treated BALB/c spleen cells (Mytomycin C treated) in the presence or absence of 100ng/ml Ksp37 protein and 500ng/ml 15K granulysin protein for 5 d. Five days after culture, CTL activity against P815 tumor cells was assessed by using 51Cr release assay.