Figures & data
Table 1. Comparison of the nucleotide and amino acid sequences of different MV vaccine strains
Figure 1. Phylogenetic analysis of MVbv. Sequences of different MV strains were aligned with ClustalW and a phylogram was generated by the neighbor-joining method using the parental MV Edmonston sequence as an out-group. The scale (0.0002) indicates the number of substitutions per nucleotide.
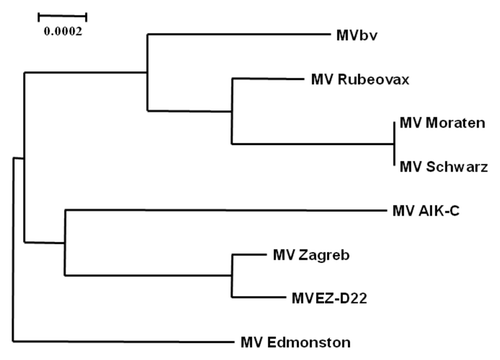
Figure 2. Comparison of the cloned MVb with different MV vaccine strains and a recombinant virus. (A) Growth kinetic analyses. Comparison of the propagation kinetics of the standard MVbv, cloned MVb and the MVEZ virus on MRC-5. Cell-free virus preparations from infected cells were collected at daily intervals and virus titers were determined by plaque assay. (B) Phenotypic assessment of various MV vaccine strains (MVbv, MVb, MVEZ, MVsch, rMVb and standard MV). Vero cells were infected, incubated at different temperatures, and the phenotype was evaluated according to the cytopathic effect. (C) Transgenic mice expressing human CD46 were immunized i.m with 1 × 105 pfu rMVb MVbv, MVEZ and MVsch. Measles end point titers are shown on a logarithmic scale.
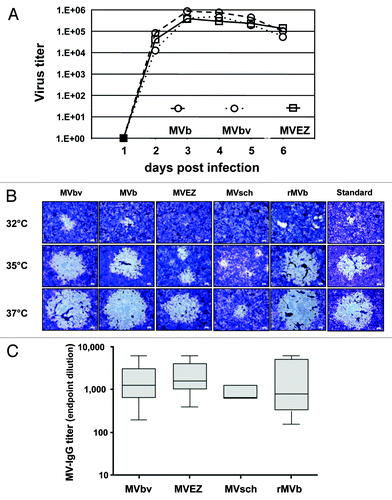
Figure 3. Transgene expression. Expression of the transgene was assessed by (A) immunofluorescence. Recombinant MV expressing HIV env (see M&M) was passaged 10 time on MRC5 cells, the resultant virus shed from passage 1 and 10 were analyzed by immunofluorescence, Dapi staining and phase contrast (Phc). Only positive expressing HIV env were formed by passaged viruses, and only negative syncytia by the control MVb (> 100 syncytia were counted). Images show typical syncytia formed by passaged viruses. (B) Antigen expression by rMVb was analyzed by western blots against HIV-env and measles N and P protein are shown. Note: mature HIV env (gp-140) is detected in the medium because it is a secreted protein. In the cell, different forms of the protein are revealed due to various maturation stages of the glycoprotein.
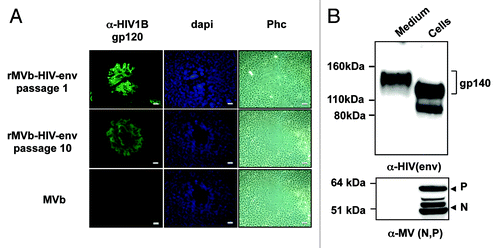
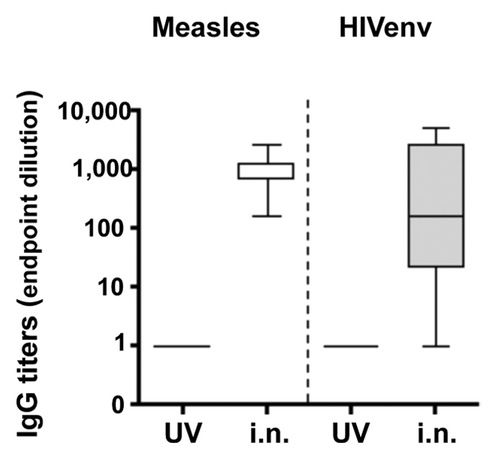
Figure 4. Induction of humoral immune responses upon intranasal application. To test the ability of rMVb vectors to induce a humoral immune response against HIV-env and MV-specific antigens, respectively, rMVb2-HIV-env was applied at 1 × 105 pfu i.n; a UV-inactivated preparation was used as control. Anti-measles and anti-HIV-env antibody titers, respectively, are represented as in .