Figures & data
Figure 1. Antigen-specific proliferative responses to T1, T2, T3, T4, T1a+T2, T2+T3 and T3+T4 OMP26 peptides from OMP26-primed and naïve DA rats. Splenic lymphocytes from immunized and non-immunized rats were cultured with individual OMP26 peptides or Concanavalin A (positive control) at concentrations of 10 μg/ml and 1 μg/ml for 3 d. Proliferative responses to peptides at 10 μg/ml are shown and values presented are expressed as mean counts per minute (CPM) ± standard deviation. Background lymphoproliferative responses to the negative E. coli protein control have been subtracted. Significance shown as ***p < 0.001, ** p < 0.02, * p < 0.05 compared with naïve lymphocytes stimulated with the same peptide on logCitation10 transformed data.
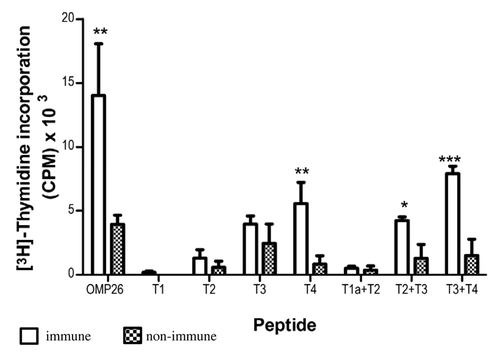
Figure 2. Lymphoproliferative responses induced by OMP26 protein by rat lymphocytes obtained after immunization with OMP26 peptides. Lymphocytes from immune and non-immune rats were stimulated with whole OMP26 protein (10 μg/ml). Background lymphoproliferative responses to the negative E. coli control have been subtracted. Only lymphocytes from animals immunized with the T3+T4 peptide demonstrated a significant response to OMP26 protein stimulation compared with non-immunized rats (**p < 0.02). The OMP26 control showed a significant response (***p < 0.001). Values presented are expressed as mean counts per minute (CPM) ± standard deviation. NI = non immune.
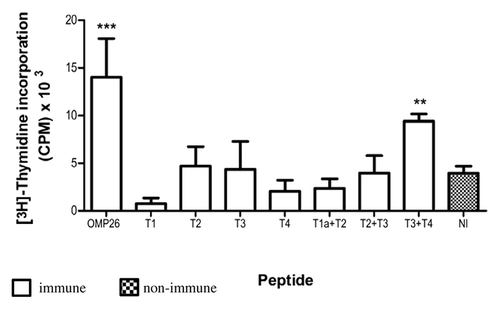
Table 1. Antibody reactivity of rat anti-OMP26 peptide against OMP26 protein and peptides
Figure 3. Binding of anti-OMP26 peptide sera to intact NTHi-289 cells. The x axes represent the levels of fluorescence and the y axes represent the number of cells counted. Intact bacteria were incubated with a 1:50 dilution of antisera, stained with Alexa Fluor® 488 goat anti-rat IgG and analyzed for intensity of green fluorescence by Flow Cytometry. The gray areas represent fluorescence in the absence of antibody. Blue lines represent the negative control of bacterial cells incubated with non-immune sera. The results with each immune sera are indicated in red. The total population analyzed was 8 × 104 bacteria.
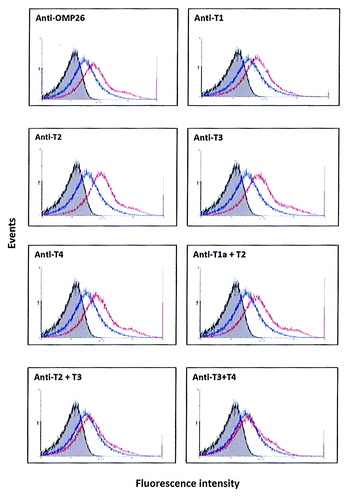
Figure 4. Antibody reactivities to the OMP26 peptides of sera from mice immunized with OMP26 protein using the IPP/IT (Panel A, C) and IP (Panel B, D) immunization regimens and assayed by ELISA (Panel A, B) and SPR (Panel C, D). Immune and non-immune sera collected from 3–5 mice were pooled and assayed in duplicate for antibody binding against the whole OMP26 protein and peptides. Antibody reactivities are expressed as IgG concentration in μg/ml in ELISA and Resonance Units (RU) in SPR. No specific antibodies to OMP26 peptides were detected in non-immune sera at the lowest sample dilution (1:25) by ELISA.
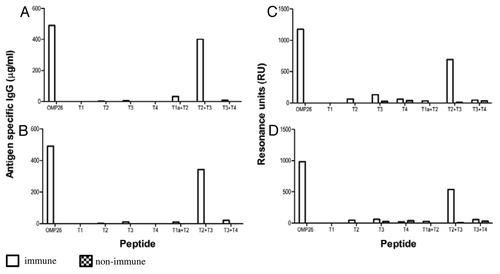
Figure 5. Antibody reactivities to the OMP26 peptides of sera from rats immunized with OMP26 protein and non-immune DA rats, using the IPP/IT (Panel A, C) and IP (Panel B, D) immunization regimens and assayed by ELISA (6A, B) and SPR (Panel C, D). Immune and non-immune sera collected from 3–5 rats were pooled and assayed in duplicate for antibody binding against the whole OMP26 protein and peptides. Antibody reactivities are expressed as IgG concentration in μg/ml in ELISA and Resonance Units (RU) in SPR. No specific antibodies to OMP26 peptides were detected in non-immune sera at the lowest sample dilution (1:25) by ELISA.
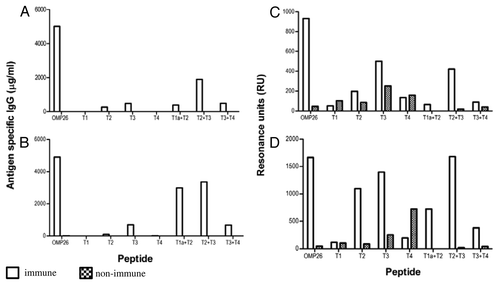
Figure 6. Antibody reactivities to the OMP26 peptides of sera from chinchillas immunized with OMP26 protein. Pooled immune and non-immune sera were tested against all OMP26 peptides immobilized on the biosensor chips and assayed by SPR. Antibody reactivities are expressed as Resonance Units (RU).
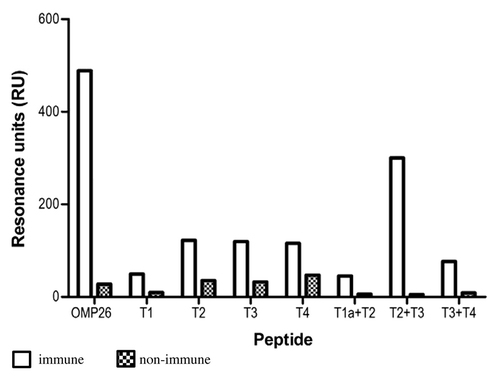
Figure 7. Summary of relative reactivity score was calculated against the OMP26 response. The OMP26 response was assigned a relative reactivity score of 5 and the responses of the other OMP26 peptides were scored against the OMP26 reactivity such that a response of 10–20% was scored as 1, 20–40% as 2, 40–60% as 3, 60–80% as 4, 80–100% as 5.
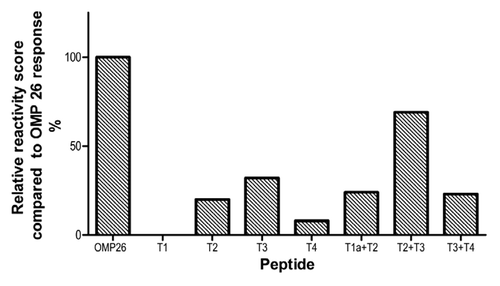
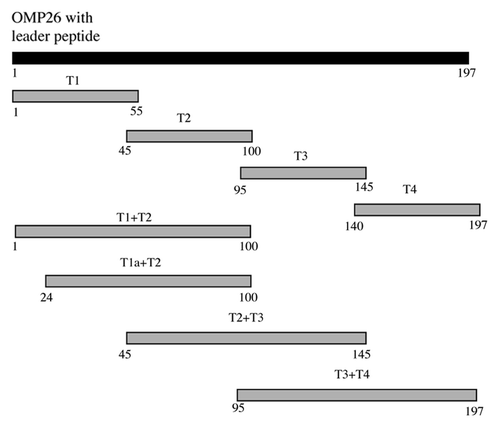
Figure 8. Schematic diagram of OMP26 peptides. Numbering is based on the translated amino acid sequence of the full-length OMP26 from NTHi-289.