Figures & data
Figure 1. (A) Schematic representation of construct encoding epitope vaccine p3Aβ11-PADRE. (B) p3Aβ11-PADRE induces anti-Aβ antibody responses in all immunized rabbits. Antibody responses were analyzed in individual sera after 2nd, 3rd and 4th immunizations by ELISA. Lines indicate the mean (n = 14). (C) All animals immunized two times with p3Aβ11-PADRE produced anti-Aβ antibodies of IgG isotype. IgG and IgM isotypes of antibodies were analyzed in individual sera of immunized animals at dilution 1:200. Error bars indicate SD (n = 14).
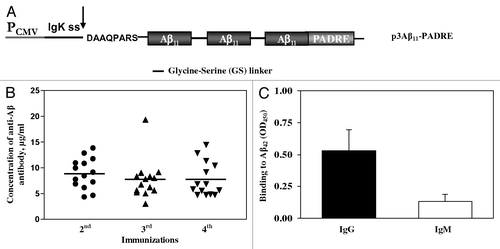
Figure 2. (A) Schematic representation of third generation epitope vaccines. Parental construct (p3Aβ11-PADRE) was modified to express protein composed of three Aβ11 B cell epitopes and nine different foreign Th cell epitopes each separated by a small glycine-serine spacer. In addition, extra amino acids between signal sequence and the Aβ11 was removed to generate protein with free N-terminal aspartic acid after cleavage of signal sequence. (B and C) Correct cleavage of signal sequence and generation of free N-terminus aspartic acid in a first copy of Aβ11 in N-3Aβ11-PADRE-Thep was analyzed in conditioned media (CM) of CHO cells transfected with p3Aβ11-PADRE-Thep (Lane 1) and pN-3Aβ11-PADRE-Thep (Lane 2) by IP/WB. Both proteins were immunoprecipitated with 6E10 MoAb. Blots were stained with 6E10 (B) or rabbit antibody specific to the N-terminus of Aβ peptide (C).
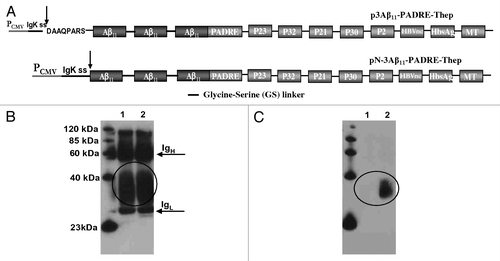
Table 1. CD4+ T cell epitopes forming the Th epitope string
Figure 3. (A) The DNA construct possessing free aspartic acid at the N-terminus and additional Th epitopes, AV-1955, induced high level of antibody after two, three and four immunizations. Lines indicate the mean (n = 9). (B) All animals immunized two times with AV-1955 produced anti-Aβ antibodies of IgG isotype. IgG and IgM isotypes of antibodies were analyzed in individual sera of immunized animals at dilution 1:200. Error bars indicate SD (n = 9). (C) Average data (mean value ± SD) of the concentration of antibodies generated in all rabbits in each group, i.e., n = 14 rabbits vaccinated with p3Aβ11-PADRE and n = 9 rabbits vaccinated with AV-1955 are presented. (D) Sera from rabbits vaccinated with either p3Aβ11-PADRE or AV-1955 bound equally to peptides possessing free or hidden N-terminal aspartic acid.
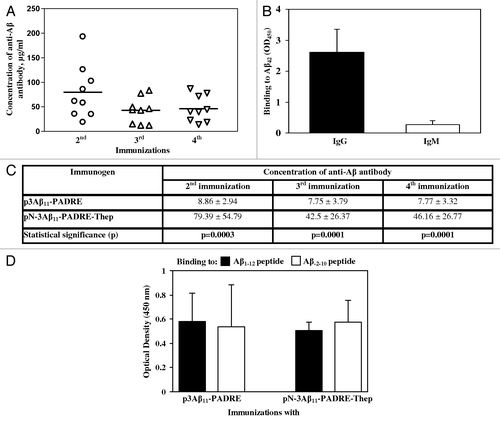
Figure 4. Rabbit anti-Aβ11 antibodies bind to Aβ42 monomeric, oligomeric, or fibrillar forms as measured using the Biacore. Different species of Aβ42 peptides were immobilized on the surface of biosensor chip CM5 and purified rabbit anti-Aβ11 antibody were run over each immobilized form of peptide. The kinetics of binding/dissociation was measured as change of the SPR signal using BIAevaluation 4.1.1 software. The gray dots represent individual data points, while the black lines represent fitted curves.
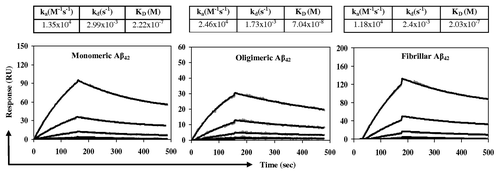
Figure 5. Rabbit anti-Aβ11 antibodies inhibit Aβ42 fibrils- and oligomer-mediated neurotoxicity. Human neuroblastoma SH-SY5Y cells were incubated with Aβ42 oligomers and fibrils, in the presence or absence of anti-Aβ11 antibody or irrelevant rabbit IgG. Control cells were treated with the vehicle, and cell viability was assayed in all cultures using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data were collected (four replicates) and were expressed as percentages of control ± s.d.
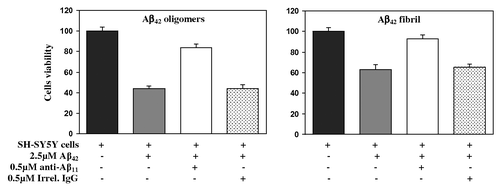
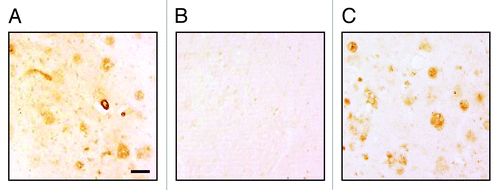
Figure 6. (A) Rabbit immune sera generated after three immunizations with AV-1955 (at dilution 1:250) bound to the 40 μm brain sections of cortical tissues from a severe AD case. (B) Binding of sera to amyloid plaques was blocked by pre-absorption of the sera with 2.5 μM Aβ42 peptide. (C) Anti-Aβ MoAb, 6E10 was used as a positive control. The original magnification is 10× and the scale bar is 100 μm.