Figures & data
Table 1. Patient characteristics
Figure 1. TCR repertoire of patient 3 showing a minimal clinical response. PBMC of patient 3 were analyzed before (upper panel) and after vaccination (middle and lower panel) for clonally expanded T cell populations by TCRβ-PCR. Fluorescent fragment analysis profiles of “reaction 1“ show two dominant clonal peaks (*) with identical fragment length, which increase during follow-up. Green peaks indicate amplification of rearrangements involving Jβ1 gene segments; blue peaks indicate amplification of rearrangements involving Jβ2 gene segments; red peak: size marker.
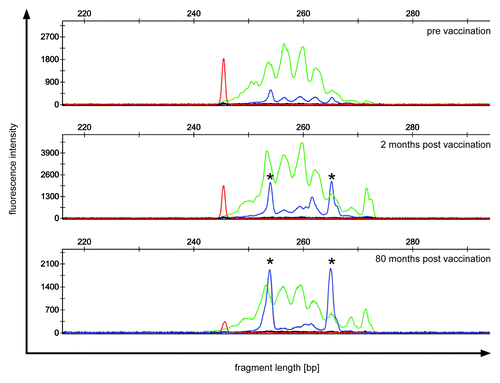
Figure 2. Immunohistology of skin biopsies from DTH challenge sites (patient no.3). (A) HE staining: a perivascular infiltrate is observed in the dermis. (B) CD3 staining. The infiltrate consists mainly of CD3 positive T-lymphocytes. (C and D) Giemsa staining showing eosinophilic infiltration
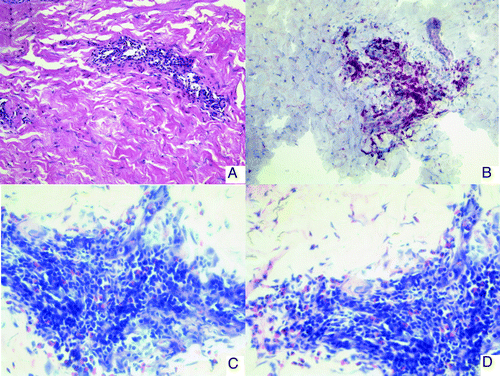
Figure 3. IFN- γ ELISpot in patients no. 3 and 7. PBMC from patients before vaccination and at different time points after vaccination were stimulated in vitro with different tumor-associated peptides from survivin, vimentin, ADFP, TYMS, cyclin-D1, c-Met, ILGF and G250. Peptide-specific T cell responses were detected by IFN- γ ELISpot. In Patient no. 7, data from TP2 were not available due to disease progression before this time point. TP, time point.
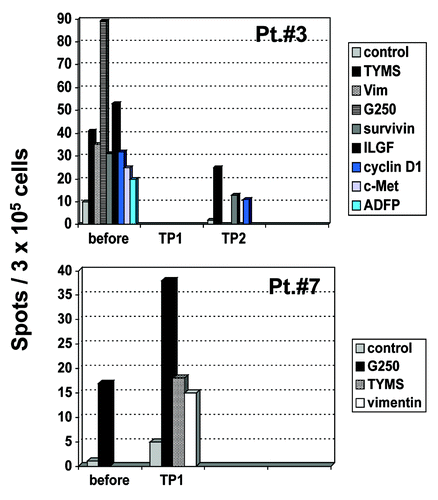
Figure 4. Cytometric bead array in immunized patients before and during vaccination. PBMC from patients before vaccination and at different time points during vaccination were stimulated with different tumor-associated peptides (survivin, vimentin, ADFP, TYMS, cyclin D1, c-Met and G250). Peptide-specific cytokine secretion was measured by CBA. The TH-polarization (TH1 vs. TH2) of the cytokine profile is indicated for each time point and for each peptide. Grey field = TH1 profile, white field = TH2 profile. Borderline = did not meet predefined criteria for a peptide-specific response. NA, not available; TP, time point.
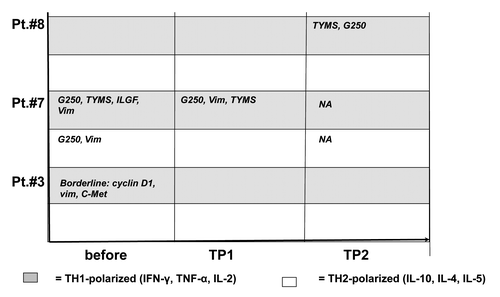
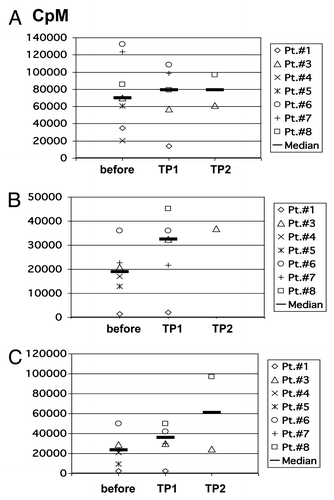
Figure 5. Proliferative capacity of patients’ PBMC. Proliferative capacity of PBMC from patients before vaccination and at different time points after vaccination in response to (A) PHA/IL-2; (B) irradiated allogeneic, partially HLA-matched unloaded DC; (C) irradiated allogeneic, partially HLA-matched DC which were loaded with autologous tumor lysate. CpM, counts per minute; TP, time point.