Figures & data
Figure 1. HI titers to TIV strains in mice and guinea pigs. CD-1 mice (A–C) or Dunkan-Hartley guinea pigs (D–F) received two injections at a 3 week interval with the indicated TIV doses with (solid bars) or without (open bars) 900 μg Vaxfectin® (“Vax”) prepared fresh at Time 0 (open and solid black bars) or after 6 mo of storage at 2–8°C (open and solid gray bars, also denoted as “-6” on x-axis labels). The y-axis depicts the GMT (+/− 95% confidence intervals for A–C) of HI antibodies measured from serum collected at week 6 (3 weeks after the second dose). Each of the three rows represents one of the three vaccine strains used to measure HI titers, indicated above each graph.
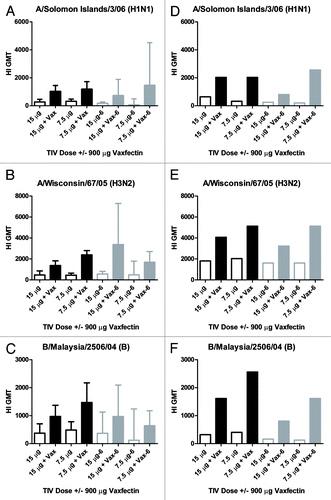
Table 1. Geometric mean titer (GMT) ratios of hemagglutination inhibition antibodies
Table 2. Time course of single radial immunodiffusion recoveries of HA in TIV
Figure 2. HI titers to different doses of TIV in mice. CD-1 mice were injected as described in legend with the indicated doses of TIV (0.0048–3 μg of each HA) adjuvanted with (black lines) or without (gray lines) 900 μg Vaxfectin®. Significantly higher responses (p < 0.05) were observed for the three highest dose groups.
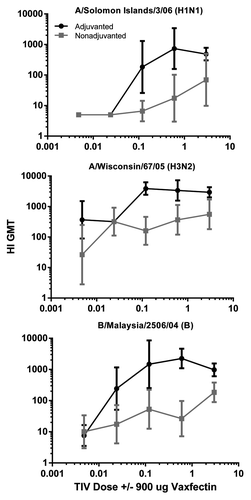
Table 3. Concentrations of serum IgG isotypes elicited by TIV +/− Vaxfectin®
Figure 3. HI titers to monovalent H5N1 vaccine in mice and guinea pigs. CD-1 mice (A) or Dunkan-Hartley guinea pigs (B) received two injections at a 3 week interval with the indicated doses of a whole virus A/Vietnam/1203/04 (H5N1) vaccine with (solid bars) or without (open bars) 900 μg Vaxfectin® freshly prepared (Time 0, open and filled black bars) or after 6 mo of storage (open and filled gray bars).
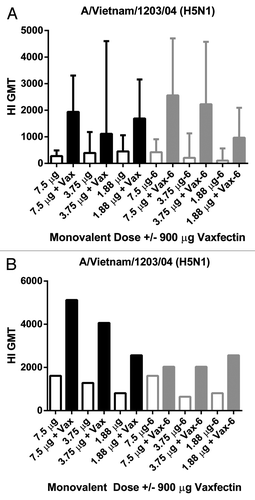
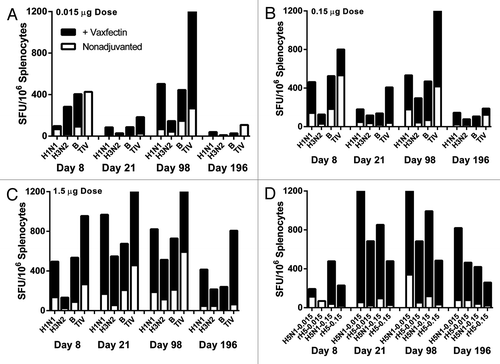
Figure 4. Frequency of vaccine-specific IFN-γ-producing T cells. BALB/c mice received two injections at a 3 week interval of the indicated TIV doses with (solid black bars) or without (open bars) Vaxfectin® and an ex vivo IFN-γ ELISPOT assay was used to measure the number of spot forming units (SFU) of IFN-γ T cells per 106 splenocytes harvested at 8, 28, 98, and 196 d after first injection. The TIV doses included 0.015 μg (A), 0.15 μg (B), and 1.5 μg (C) per each HA or the former two for the H5N1 vaccine (D). The rare instances of open bars only (e.g., upper left panel, Day 8 TIV and Day 196 TIV) indicate the adjuvanted response did not exceed the nonadjuvanted response. The sources of antigen for recalling H5-specific responses included H5N1 and recombinant H5 HA (“rH5”). Due to the large number of treatment groups tested, each bar represents the pooled splenocytes of each group; therefore no statistical analyses were possible. The TIV data were derived from two separate studies (0.015 μg and 0.15 μg doses in one study and 1.5 μg dose in a second study).