Figures & data
Figure 1. ACAM2000® Blackbox warning.Citation12

Figure 2. Case definitions adapted from the smallpox vaccine adverse reactions surveillance guidelinesCitation11 and Brighton Collaboration Vaccinia Virus Vaccine Adverse Events Working Group.Citation31aExamples include: erythema multiforme, urticaria, non-viral pustulosis, viral exanthem. bConjunctivitis: inflamed membrane that lines the inner surface of the eyelid and exposed surface of the eyeball excluding the cornea with serous or mucopurulent discharge and the presence of vesicles, ulcerations, or “moist appearing” white lesions. cBlepharitis: pustules, edema, hyperemia of eyelid +/− lymphadenopathy (periauricular or submandibular), cellulitis, fever. dPeriocular area: papules, vesicles or pustules above the brow or below the inferior occipital rim, but not involving adnexa, lids, lid margins or canthi
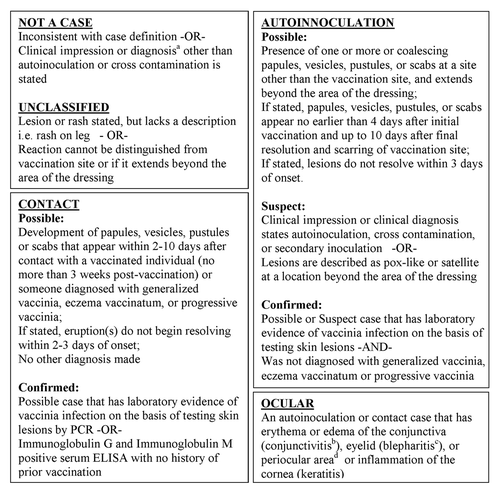
Figure 3. Flow diagram illustrating selection, exclusion, and classification of VAERS reports that were reviewed as potential cases of unintentional transfer of vaccinia.