Figures & data
Table 1. Characteristics of the study population
Table 2. Characteristics of the specific vaccination regimes
Figure 1. Distribution of humoral responses to HBsAg in vacinees immunized when ≤ 35 y of age and ≥ 55 y of age. Antibody responses were categorized as non-response (NR) when titers were < 10 IU/L, intermediate response (IR) for titers 10–999 IU/L, or high response (HR) for titers ≥ 1000 IU/L. Application of a χ2-test demonstrated a significant difference (p = 0.046) between age at the time for the primary vaccination and the degree of antibody response.
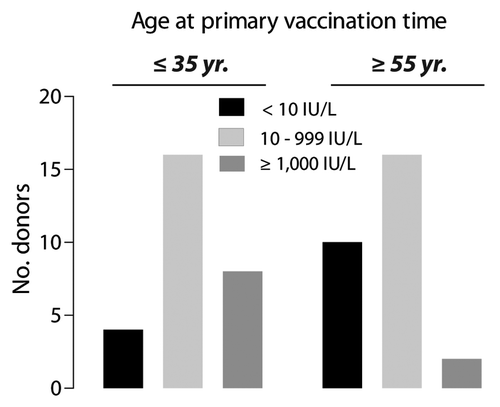
Table 3. Antibody response following primary and booster vaccination of vaccinees with low (≤ 20 IU/L) or no protective antibody response
Figure 2. Proliferative recall response to HBsAg (10 µg/ml) tested by in vitro stimulation of PBMCs from HBV-vaccinated individuals. The donors were ≤ 35 y (n = 28), or ≥ 55 y (n = 27) when they received the primary immunization course. The stimulation ratio (SR) expresses the ln-transformed ratio of the antigen-specific response normalized to the polyclonal stimulation of T cell proliferation with antibodies to CD28 and CD3 according to Equation 1 (defined in the Materials and Methods). To compensate for right skewing of the data, the cpm values are shown as ln median ± SD.
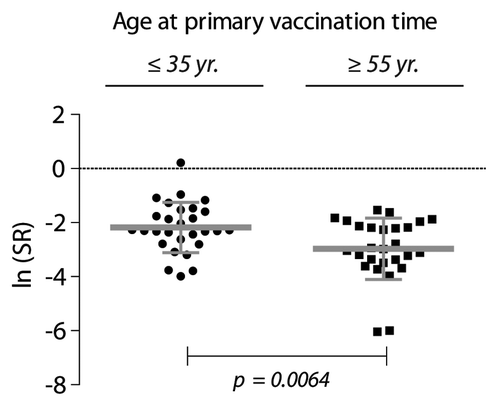
Figure 3. IFN-γ production from non-stimulated and HBsAg-stimulated PBMCs. (A and B) The mass concentration of IFN-γ (ρ) was measured in supernatant from cultured cells 48 h-post stimulation with a final concentration of 10 μg/ml HBsAg (+HBsAg) or with no stimulation (none). Cells were derived from donors ≤ 35 y (A; n = 23) and ≥ 55 y (B; n = 19). Results for each of the donors are shown together with medians (black bars). The data were analyzed by Wilcoxon matched-pairs signed rank test. (C) Correction for IFN-γ baseline levels were performed by simple subtraction of the mass concentration of IFN-γ in supernatant from unstimulated cell cultures (ρIFN-γ, none) from the concentration obtained in HBsAg-stimulated cultures (ρIFN-γ, +HBsAg).
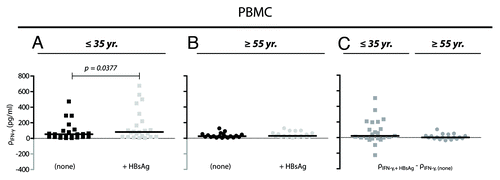
Figure 4. Gating strategy for naive and memory CD4+ T lymphocytes. The CD4+ T cell population was selected in a side scatter vs. CD4-FITC dot plot. Within this subset CD4+ T cells were divided based on their expression of CD62L and CD45RA and were identified as naïve T cells (TNaïve; CD62L+CD45RA+), effector T cells (TEffector; CD62L-CD45RA+), central memory T cells (TCM; CD62L+CD45RA-), and effector memory T cells (TEM; CD62L-CD45-).
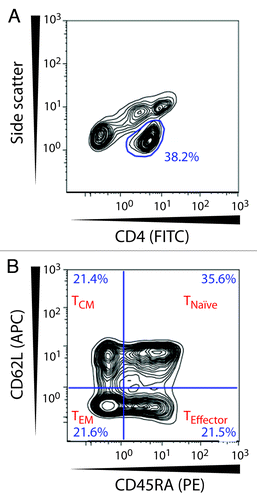
Figure 5. Association between CD62L expression levels and the humoral response to HBV vaccination. The donors were stratified into antibody response groups of non-responders (NR) (< 10 IU/L), intermediate responders (IR) (10–999 IU/L), and high responders (HR) (≥ 1000 IU/L). One-way ANOVA analyses for each age group were performed to test the relationship between CD62L expression and titers of HBsAg-specific antibodies. (A) The results are shown as a scatter plot with mean ± SD (a) for individuals ≤ 35 y [NR (n = 4), IR (n = 16), HR (n = 8)]. A statistically significant difference was found between the group mean CD62L MFI values for CD4+ TNaïve cells (p = 0.0314) and TCM cells (p = 0.0197). (B) Individuals ≥ 55 y [NR (n = 10), IR (n = 15), HR (n = 2)] showed no difference between the different response categories.
![Figure 5. Association between CD62L expression levels and the humoral response to HBV vaccination. The donors were stratified into antibody response groups of non-responders (NR) (< 10 IU/L), intermediate responders (IR) (10–999 IU/L), and high responders (HR) (≥ 1000 IU/L). One-way ANOVA analyses for each age group were performed to test the relationship between CD62L expression and titers of HBsAg-specific antibodies. (A) The results are shown as a scatter plot with mean ± SD (a) for individuals ≤ 35 y [NR (n = 4), IR (n = 16), HR (n = 8)]. A statistically significant difference was found between the group mean CD62L MFI values for CD4+ TNaïve cells (p = 0.0314) and TCM cells (p = 0.0197). (B) Individuals ≥ 55 y [NR (n = 10), IR (n = 15), HR (n = 2)] showed no difference between the different response categories.](/cms/asset/19af8c8a-a77a-46a0-9ebb-c55a81ef53fe/khvi_a_10924480_f0005.gif)
Figure 6. Gating strategy for identification of functional CD62L on CD4+ T lymphocytes. The CD4+ T cell population was selected based on side scatter and CD4-APC expression. Hereafter binding of the glycoprobe to functional CD62L molecules was distinguished by means of the fluorescent glycoprobe signal and forward scatter.
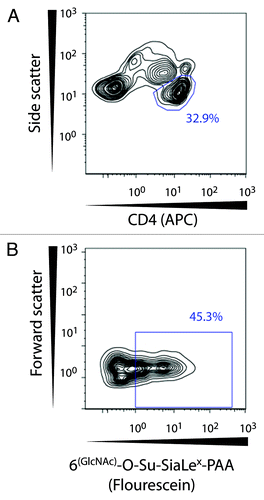
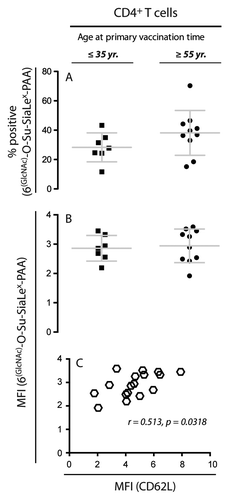
Figure 7. Flow cytometric analysis of glycoprobe-binding to CD62L+ T cells. To investigate if the expression of functional CD62L in NR donors ≥ 55 y of age could account for their inability to raise responses against HBsAg, the percentage of T cells binding the glycoprobe ligand for CD62L and the MFI for staining with the glycoprobe was compared for HR donors ≤ 35 y and NR donors ≥ 55 y. (A) Percentage of CD4+ T cells positive for staining with the glycoprobe analyzed in 17 subjects either ≤ 35 y (n = 7; anti-HBsAg titer ≥ 1000 IU/L) or ≥ 55 y (n = 10; anti-HBsAg titer < 10 IU/L) who had received the complete course of HBV vaccination. (B) Analysis of the MFI from staining with the glycoprobe after gating on CD4+ T cells. (C) Analysis of the correlation between the MFI for staining against CD62L with specific antibody and the MFI for the glycoprobe binding to CD4+ T cells.