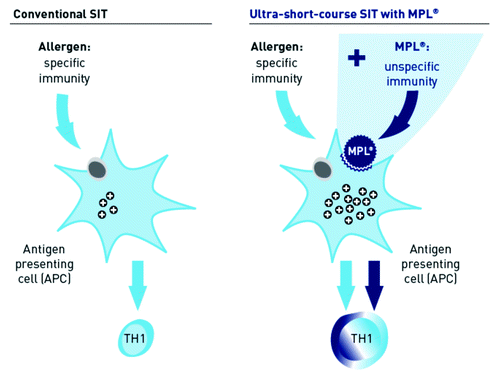
Figures & data
Table 1. Thirteen grass product
Figure 2. Basophil histamin-release in patients treated with four injections of Pollinex Quattro (left) or placebo (right) before and after treatment.Citation19
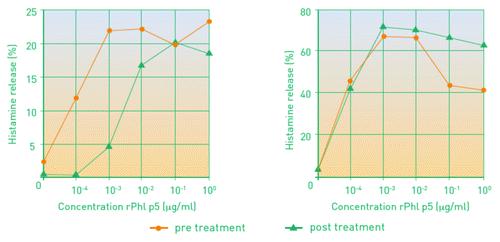
Figure 3. Leukotriene levels as a surrogate parameter of inhibiting antibodies before and after immunotherapy.Citation20
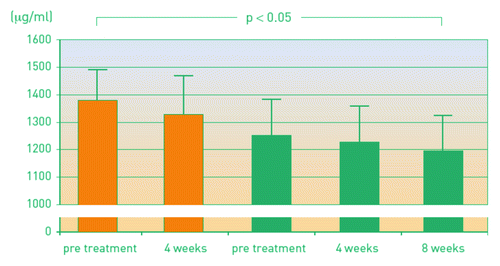
Table 2. Overview of phase 3 clinical studies and post-marketing trials with Pollinex Quattro: study design and key results
Figure 4. Combined Symptom/medication Score in the randomized, placebo-controlled phase 3 study in patients with tree pollen allergy (n = 58).Citation18
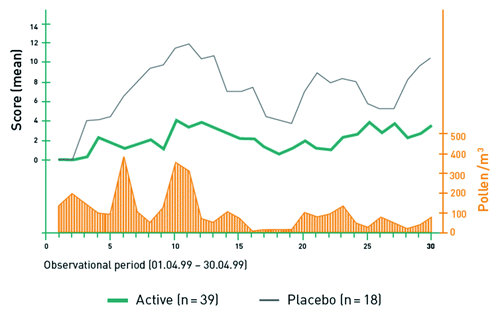
Figure 5. Proportion of well subjects; i. e. CSMS ≤ 2 during the peak pollen season (a; n = 973) and median number of well days; i.e., patients with a score ≤ 2 and no anti-allergic medication (b; n = 990) in the randomized, placebo-controlled phase 3 study.Citation26
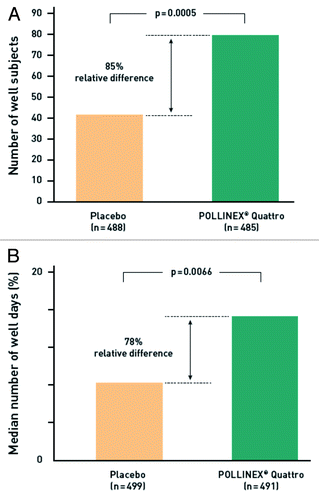
Figure 6. Use of symptomatic medication during therapy in 324 patient completing 3 y of immunization.Citation28
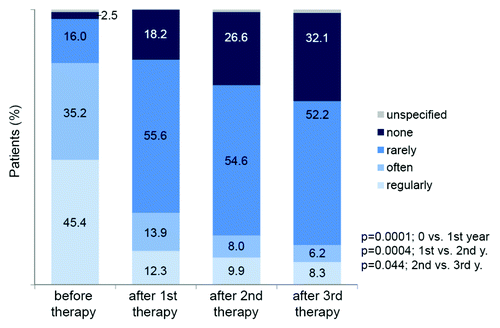
Table 3. Summary of local adverse events in clinical trials during the treatment period
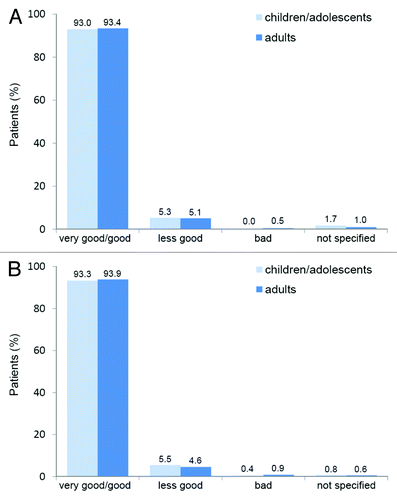
Figure 7. Therapy tolerability (a) and acceptance (b) in adults (n = 2,692) and children/adolescents (n = 422).Citation28