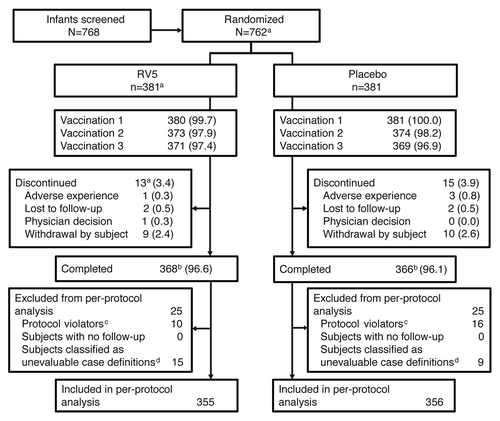
Figures & data
Figure 1. Study disposition. Data are represented as n (%) unless otherwise noted. aIncludes 1 subject who was randomized but did not receive the study vaccine by the investigator’s decision. bCompleted: the number of subjects who continued follow-up until the last study visit (visit 5) regardless of the number of vaccinations received. cSubjects who had <3 vaccinations or <28 d between vaccinations, who received oral poliovirus vaccine or Bacille Calmette-Guérin within 27 d of any dose. dSubjects were classified as unevaluable owing to wild-type rotavirus-positive test results prior to 14 d post dose 3, incomplete clinical and/or laboratory results or stool samples collected out of the day range (e.g., within 7 d after the onset of symptoms).