Figures & data
Figure 1. Characterization of the purified rVP6 and dl2/6-VLPs. (A) Purity and integrity analysis of RV rVP6 (lane 1) and dl2/6-VLPs (lane 2) with SDS-PAGE followed by PageBlue staining. Lane M illustrates molecular weight marker. Corresponding molecular weights are indicated with arrows on the right of the gel image. (B) Electron microscopy images of morphological structures assembled by RV rVP6 (panel 1) and dl2/6-VLPs (panel 2) corresponding to the SDS-PAGE lanes 1 and 2 (A) respectively. Protein structures were examined after negative staining with 3% uranyl acetate pH 4.6. (C) Evaluation of antigenicity of the purified rVP6 and dl2/6-VLPs at different concentrations with ELISA for total IgG antibodies using human polyclonal anti-rotavirus serum. Mean OD values with standard errors of duplicate wells are shown.
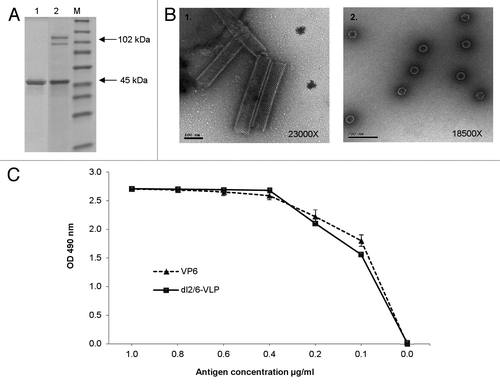
Figure 2. Humoral immune response in BALB/c mice immunized with RV VP6 proteins. (A) Kinetics of RV VP6–specific total IgG antibodies in sera of BALB/c mice (3–5 mice/group) immunized intramuscularly with 3 μg or 10 μg doses of rVP6 or dl2/6-VLPs at weeks 0 and 3. Group means with standard errors of tail blood samples collected at study weeks 0 (pre-immune sera) and 3 and termination sera at week 5 are shown. (B) End point serum titrations of RV VP6-specific IgG antibody responses of different experimental groups of mice. Control mice receiving carrier only (PBS) were used as controls. Mean titers with standard errors of termination sera at week 5 are shown. A dashed line indicates the lower cut off value (OD 0.15) calculated as follows: mean OD (termination sera of control mice) + 3 × SD (C) VP6-specific IgG1 and IgG2a subtype antibody responses of groups of mice immunized with rVP6 or dl2/6-VLPs. Data are expressed as the geometric mean titers (log10) with standard errors of the reciprocal dilutions of specific IgG1 and IgG2a antibodies in termination sera at week 5.
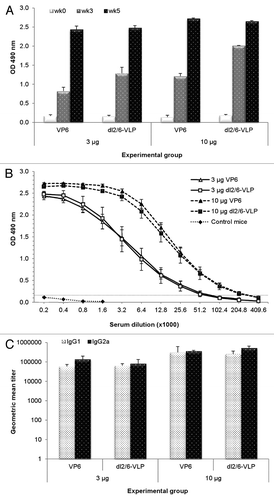
Figure 3. Cross-reactivity of rVP6 induced serum IgG antibodies to several RV strains in mice immunized twice with 10 μg of rVP6 or dl2/6-VLPs. Control mice received carrier only (PBS). Mean ODs (490 nm) of the experimental groups with standard errors are shown.
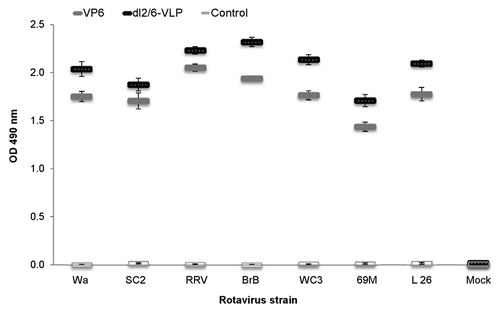
Figure 4. Rotavirus VP6-specific T-cell response detection by ELISPOT. (A) Different RV cell cultures and a synthetic RV R6–2 peptide were used to stimulate interferon-γ (IFN-γ) production from the splenocytes of mice immunized twice with 3 μg rVP6 or dl2/6-VLPs. Control mice received carrier only (PBS). Results are expressed as mean IFN-γ spot forming cells (SFC)/106 cells of duplicate wells with standard errors. (B) CD4+ T cells are responsible for IFN-γ production of mice immunized twice with 10 μg dl2/6-VLPs. Splenocytes were stimulated with different RV cell cultures and a synthetic R6–2 peptide in the presence or absence of CD4 and CD8 specific antibodies or control antibodies to block the T cell activation. Results are expressed as the mean % inhibition of duplicate wells.
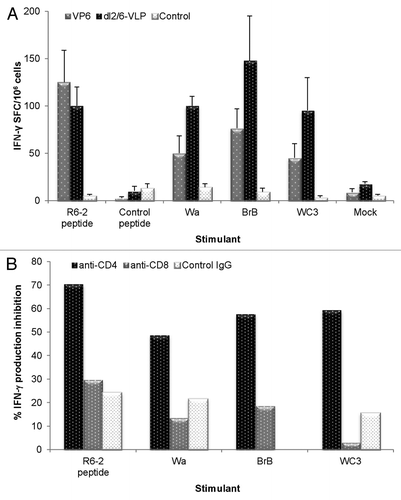
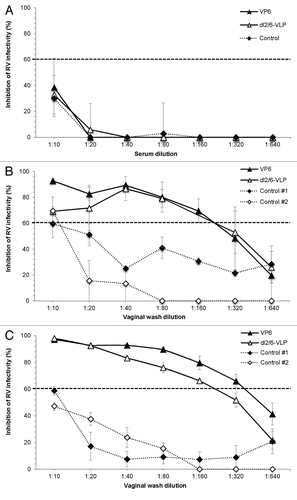
Figure 5. Inhibition of rotavirus infection in vitro. Inhibition activity of sera (A) and vaginal washes (B and C) of mice immunized with 10 μg of rVP6 or dl2/6-VLPs against homologous rotavirus Wa (A and B) and heterologous RRV (C) was analyzed by Neutralizing ELISA (NELISA). Sera and vaginal washes of mice receiving carrier only (PBS) were used as controls. Control #1 and #2 shown in panels B and C represent two separate pooled controls. In neutralization experiments the immunized and control samples from the same time point (week 5) were run simultaneously. Only neutralization of control #2 against Wa in panel B has been run later. Results are shown as the mean % inhibition of rotavirus infectivity of at least two independent experiments, each done in duplicate, with standard errors. A dashed line indicates 60% reduction in virus infectivity.