Figures & data
Table 1. Baseline characteristics of study participants
Figure 1. Prevaccination geometric mean titers and seroprotection rates. (A) Geometric mean titers (GMTs) for the 18−59 y age groups at day 0. (B) GMTs for the ≥ 60 y age groups at day 0. (C) Seroprotection rates for the 18−59 y age groups at day 0. (D) Seroprotection rates for the ≥ 60 y age groups at day 0. ID: intradermal vaccine; IM: intramuscular vaccine.
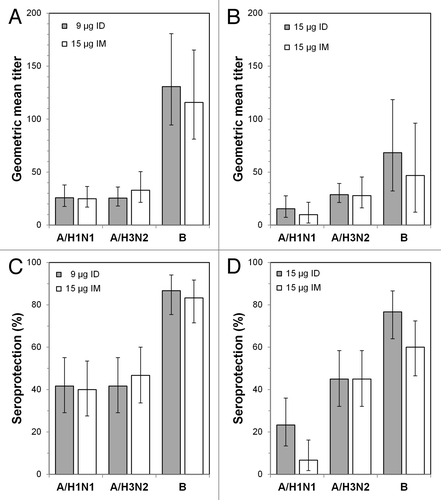
Figure 2. Immunogenicity outcomes after intradermal (ID) or intramuscular (IM) influenza vaccination in the 18−59 y age group. Subjects were vaccinated with 9 μg ID vaccine or with 15 μg IM vaccine, and immunogenicity characteristics were determined 21 d later. (A) Geometric mean titers (GMTs) at day 21. (B) GMT ratios (GMTRs), which were the geometric means of the day 21 hemagglutinin inhibition (HI) titer divided by the pre-vaccination (day 0) HI titer for each subject. (C) Seroprotection rates. Error bars indicate the 95% confidence intervals, and the horizontal bars indicate the Committee for Medicinal Products for Human Use criteria for adults 18–59 y old.
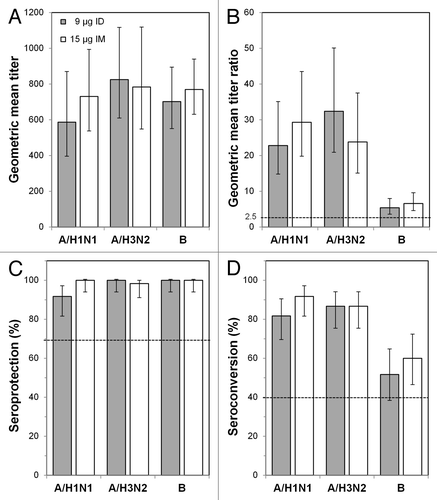
Figure 3. Immunogenicity outcomes after intradermal (ID) or intramuscular (IM) influenza vaccination in the ≥ 60 y age group. Subjects were vaccinated with 15 μg ID vaccine or with 15 μg IM vaccine, and immunogenicity characteristics were determined 21 d later. (A) Geometric mean titers (GMTs) at day 21. (B) GMT ratios (GMTRs), which were the geometric means of the day 21 hemagglutinin inhibition (HI) titer divided by the pre-vaccination (day 0) HI titer for each subject. (C) Seroprotection rates. (D) Seroconversion rates. Error bars indicate the 95% confidence intervals, and the horizontal bars indicate the Committee for Medicinal Products for Human Use criteria for adults ≥ 60 y old.
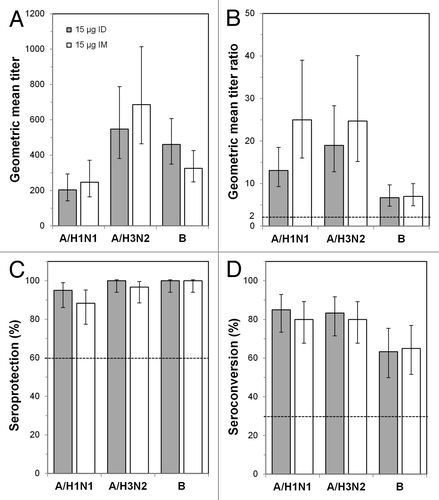
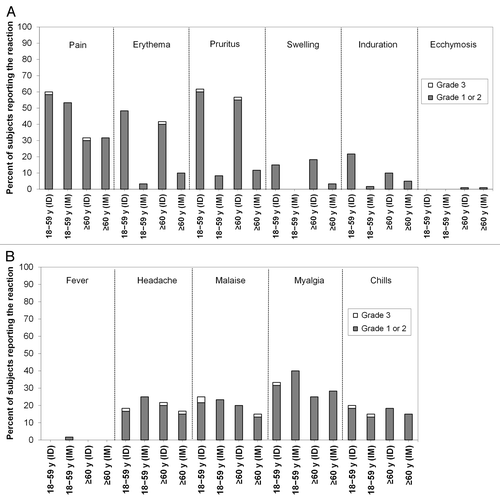
Figure 4. Solicited injection site and systemic reaction profiles. (A) Solicited injection site reactions. (B) Solicited systemic reactions. Data are the percentages of subjects within each group who experienced a grade 1 or 2 reaction or a grade 3 reaction through post-vaccination day 7. ID: intradermal vaccine; IM: intramuscular vaccine.