Figures & data
Figure 1. Most clinical DC vaccine studies to date have employed “IL-4 DCs.” These DCs are generated by in vitro differentiation (1) of monocytes for 5–6 d in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). These immature IL-4 DCs then undergo maturation (2) for 1–2 d by adding a cocktail of pro-inflammatory cytokines (A). We have previously designed an alternative protocol for the generation of monocyte-derived DCs, termed “IL-15 DCs.” These IL-15 DCs are generated by rapid (24–48 h) differentiation of monocytes using GM-CSF and interleukin-15 (IL-15), followed by maturation induction for 16–20 h through triggering of the Toll-like receptor (TLR)-7/8 signaling pathway (B).
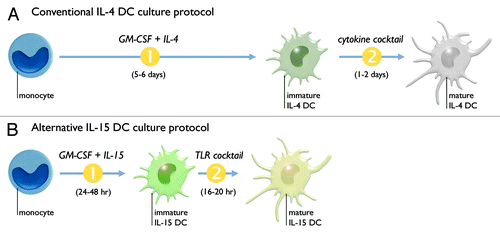
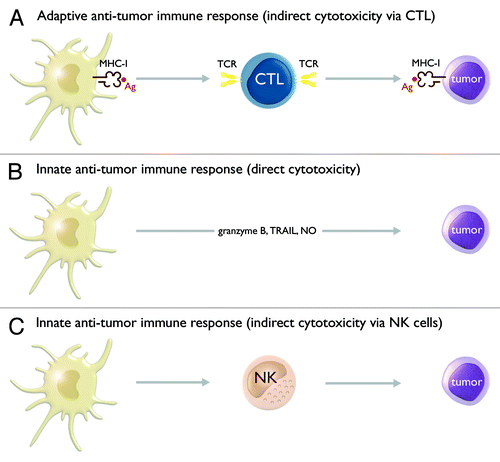
Figure 2. TLR-matured IL-15 DCs have pleiotropic immunostimulatory properties, which allow them to harness both adaptive and innate anti-tumor immune responses. At the level of the adaptive anti-tumor immune response, IL-15 DCs display a potent capacity to present tumor antigen (Ag) in the context of major histocompatibility complex class I (MHC-I) molecules to CD8+ cytotoxic T lymphocytes (CTLs). These CTLs recognize MHC-I/Ag-expressing tumor cells via their T cell receptor (TCR) and subsequently mediate tumor cell lysis (A). At the level of the innate anti-tumor immune response, IL-15 DCs can mediate tumor cell lysis through a direct cytotoxic action. Several mechanisms have been implicated in IL-15 DC-mediated cytotoxicity, including granzyme B, tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) and nitric oxide (NO) (B). In addition, IL-15 DCs can also indirectly contribute to innate tumor destruction via activation of natural killer (NK) cells (C).