Figures & data
Figure 1. HI antibody responses in serum. (A) HI antibody responses before and after primary and secondary vaccination are shown for serum. A paired t test was performed to compare data from week 0 (pre) and 6 (post). Correlation coefficient (r) and p value were calculated. *; p < 0.05. (B) The relationship of serum HI antibody responses before and after secondary vaccination. The abscissa and ordinate show the pre- and post-vaccination HI titers, respectively. Further, it is shown how these relate to conversion rate and protection rate, which are on the border or within the area marked by the bold line and by the light gray background, respectively. Each circle represents an individual and shows the relation between the pre- and post-vaccination titers. Gray circles indicate subjects between 60 and 69 y-of-age.
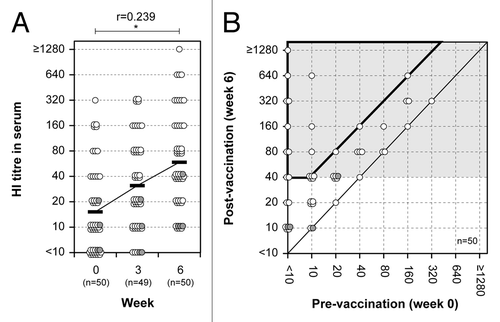
Table 1. Tools currently in use to evaluate vaccine-induced changes in serum HI titer
Table 2. Serum HI antibody responses after two doses of the nasal A/Victoria vaccine
Figure 2. Neutralizing antibody responses and correlation between HI and NT titers in serum. (A) Neutralizing antibody responses before and after primary and secondary vaccination are shown. A paired t test was performed to compare data from week 0 (pre) and 6 (post). The correlation coefficient (r) and p value were calculated. **; p < 0.01. (B) Correlation between HI and NT titers in serum 3 weeks after the secondary nasal vaccination. The abscissa and ordinate show HI and NT titers, respectively. Pearson r value and p value were calculated. Each individual is represented by a circle showing corresponding HI and NT titers. Gray circles indicate subjects between 60 and 69 y-of-age.
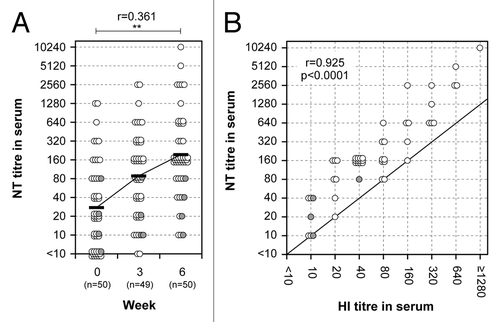
Table 3. Serum neutralizing antibody responses after two doses of the nasal vaccine
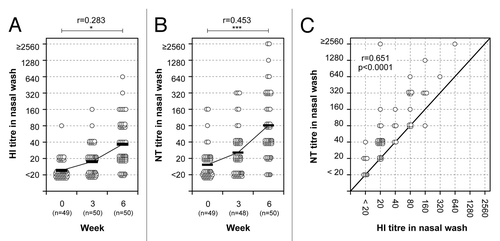
Figure 3. HI and neutralizing antibody responses in nasal mucus. HI (A) and NT (B) titers before and after primary and secondary vaccination. A paired t test was performed to compare data from week 0 (pre) and 6 (post). The correlation coefficient (r) and p value were calculated. *; p < 0.05, ***; p < 0.001. (C) Correlation between HI and NT titers in nasal wash 3 weeks after the secondary nasal vaccination. The abscissa and ordinate show HI and NT titers, respectively. Pearson r value and p value were calculated. Each circle represents an individual showing corresponding HI and NT titers. Gray circles indicate subjects between 60 and 69 y-of-age.