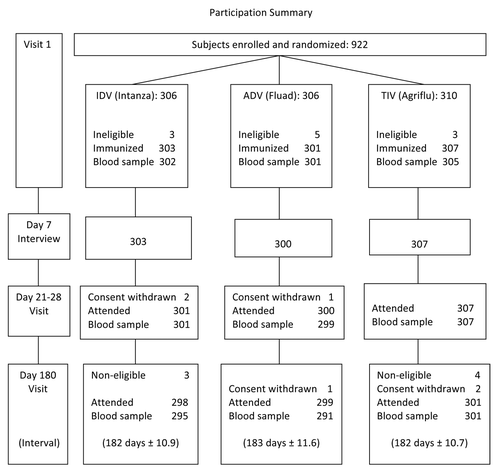
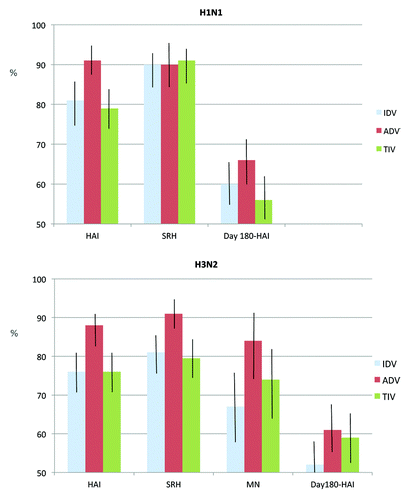
Figures & data
Table 1. Study participant demographics.
Table 2. Reported systemic adverse events prior to and following Influenza immunization
Table 3. Injection site reactions following Influenza vaccines.
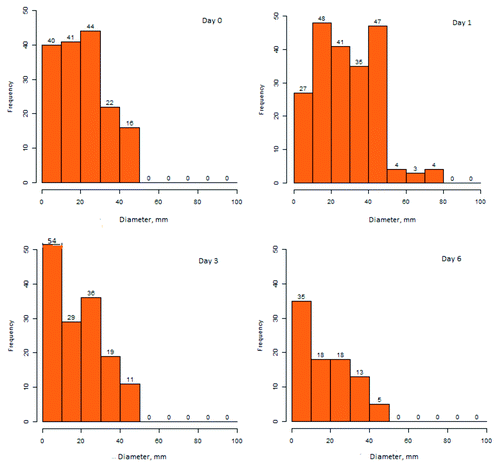
Figure 2. Frequency and diameter of erythema at IDV injection sites at intervals following immunization.