Figures & data
Figure 1. Study design. Subjects were randomly allocated in a 1:1:1:1:1 ratio to one of five treatment groups: 50, 100, or 200 µg IC43 with adjuvant, 100 µg IC43 without adjuvant, or placebo (0.9% sodium chloride) and two intramuscular injections were given in the deltoid region 7 d apart. Note: Vaccinations were given on day 0 and day 7. R, randomization; w/o adj, without aluminum hydroxide adjuvant. aPlacebo = 0.9% sodium chloride.
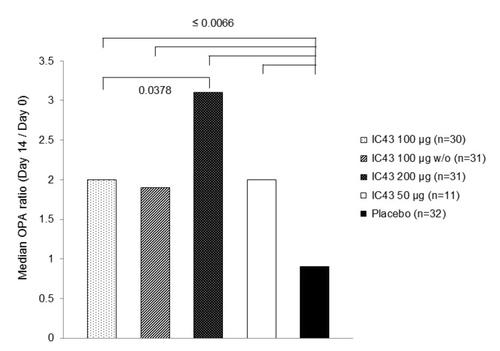
Figure 2. OprF/I-specific IgG antibody geometric mean titer by treatment group (per protocol population). The primary immunogenicity analysis of OprF/I-specific IgG antibody titer on day 14 (7 d after the second vaccination) demonstrated significant differences between treatment groups (P < 0.0001) with a significant study center effect (P = 0.0200) in the per protocol population and a non-significant study center effect (P = 0.0716) in the intention to treat population. Note: Statistically significant P values for pairwise comparisons of GMT ratio estimates are shown (analysis of variance with treatment group and study center as fixed factors, adjusted for multiple comparisons by Tukey’s Honestly Significant Difference test). D, day; GMT, geometric mean titer; IgG, immunoglobulin G; OprF/I, outer membrane protein OprF/I hybrid vaccine; w/o, without adjuvant.
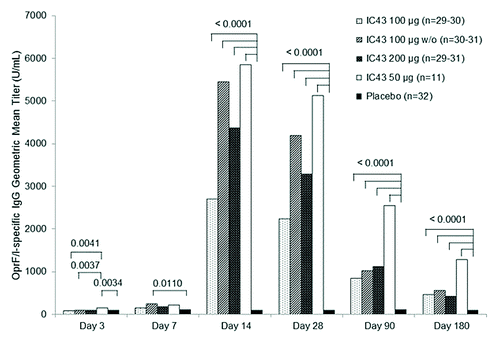
Table 1. Number and percentage of subjects with a ≥4-fold increase in OprF/I-specific IgG antibody geometric mean titer from day 0 (first vaccination) to day 14 (7 d after second vaccination) (per-protocol-population)
Table 2. Number and percentage of subjects with a ≥4-fold increase in OprF/I-specific IgG antibody geometric mean titer from day 0 (first vaccination) to day 180 (6 mo after first vaccination) (per protocol population)
Figure 3. Median OPA ratio (change in OPA titer from day 0 to day 14) by treatment group (per protocol population). From day 0 to day 14, a ≥2-fold increase in OPA titer was observed in 54.5% of all subjects who received IC43 (range 46.7%−63.3% across all IC43 treatment groups; PP population) and 1 subject (3.1%) in the placebo group. Note: P values from Wilcoxon test for unpaired observations on OPA ratio (day 14/day 0) are statistically significant for comparisons between each IC43 treatment group and placebo, and between IC43 100 μg and IC43 200 μg. OPA, opsonophagocytic assay; w/o, without adjuvant.
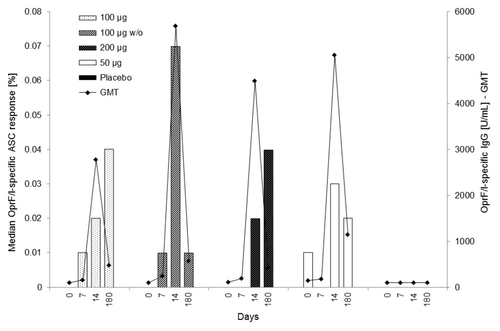
Table 3. Avidity index of OprF/I-specific IgG antibodies at day 7 and day 14 by treatment group (per protocol population; subgroup of subjects with detectable OprF/I-specific IgG antibodies at day 7 and day 14)
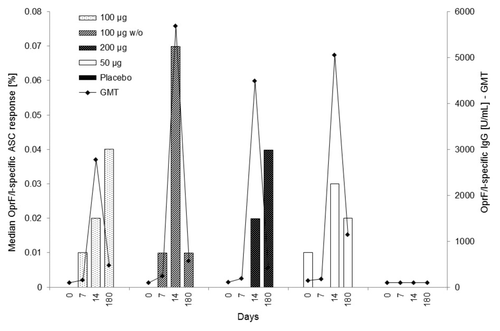
Figure 4. Median OprF/I-specific ASC response (%) vs. OprF/I-specific IgG geometric mean titer (U/mL) on days 0, 7, 14, and 180 (PP population). For median OprF/I-specific ASC response, the number of observations was n = 10 (100 μg), n = 9–10 (100 μg w/o), n = 9–11 (200 μg), n = 10–11 (50 μg), and n = 11–12 (placebo). For OprF/I-specific IgG geometric mean titer, the number of observations was n = 30 (100 μg), n = 30–31 (100 μg w/o), n = 30–31 (200 μg), n = 11 (50 μg), and n = 32 (placebo). OprF/I, outer membrane protein; OprF/I, hybrid vaccine; w/o, without adjuvant.