Figures & data
Figure 1. In vivo transduction of Ad4 and Ad7 vectors. Ad4 and Ad7 viruses expressing a GFPLuc fusion gene were used to immunize mice by intramuscular and intranasal routes and imaged for luciferase activity 24 h later. In vivo transduction and luciferase expression in BALB/c mice is shown (A). Intramuscular transduction of Ad7 was restored in CD46+ transgenic mice and compared with a standard replication-defective Ad5 vector expressing the same transgene (B). The sum lumenescence was determined using the lumazone analysis software and levels were compared using a 2-tailed T-Test (C). Error bars indicate standard error.
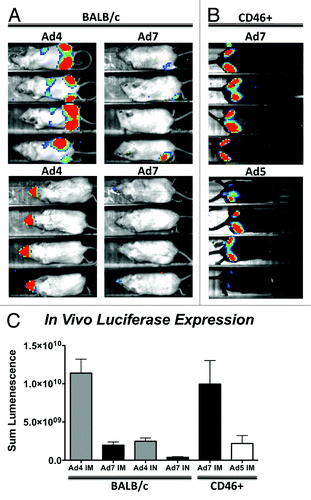
Figure 2. Protection by systemic intramuscular immunization in BALB/c mice. Groups of 5 female BALB/c mice were immunized intramuscularly with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
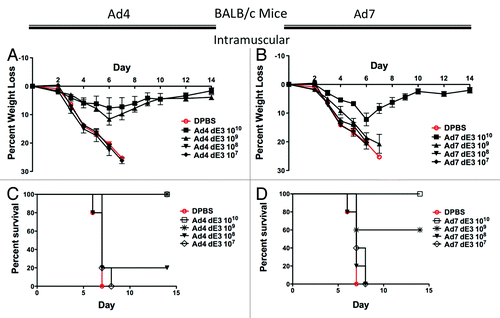
Figure 3. Protection by mucosal intranasal immunization in BALB/c mice. Groups of 5 female BALB/c mice were immunized intranasally with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
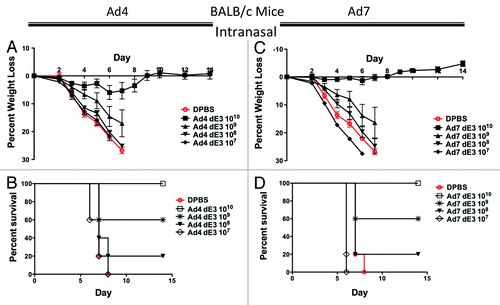
Figure 4. Protection by systemic intramuscular immunization in human CD46+ transgenic mice. Groups of 5 female CD46+ mice were immunized intramuscularly with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
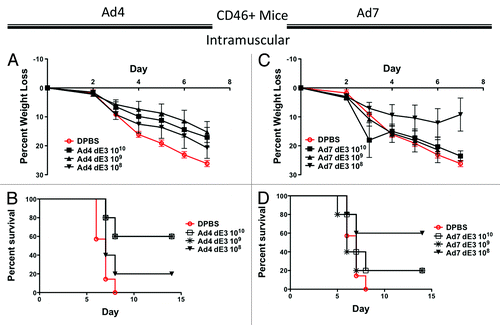
Figure 5. Protection by mucosal intranasal immunization in human CD46+ transgenic mice. Groups of 5 female CD46+ mice were immunized intranasally with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
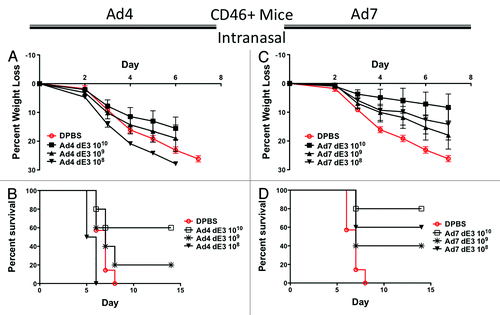
Figure 6. Schematic of Ad4 gDNA cloning. The right and left arms of Ad4 were amplified with primers designed to incorporate a unique NdeI restriction site, an overlap of ~27 nucleotides and flanking PacI restriction sites (A). The 2 PCR products were fused together in a second round of amplification (B). A schematic representation of the cloning fragment is shown (C). The cloning PCR fragment is ligated to the Kanamycin resistance gene and the pBR322 origin of replication (D). The recomb cloner plasmid was digested with NdeI and recombined with the Ad4 genomic DNA in vitro using BJ5183 electrocompetent cells (E). The complete Ad4 genomic DNA plasmid is shown (F).
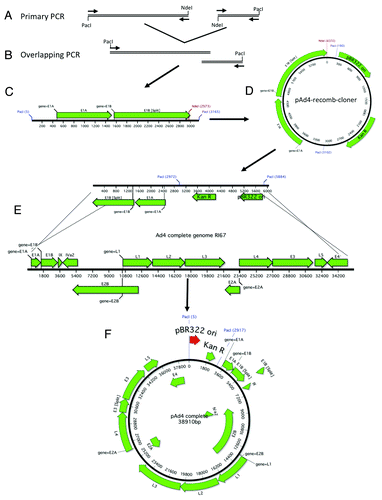
Table 1. Primers for cloning Ad4 and Ad7 gDNA
Figure 7. Schematic of Ad7 gDNA cloning. The right and left arms of Ad7 were amplified with primers designed to incorporate a unique PmeI restriction site, an overlap of ~27 nucleotides and flanking AsiSI restriction sites (A). The 2 PCR products were fused together in a second round of amplification (B). A schematic representation of the cloning fragment is shown (C). The cloning PCR fragment is ligated to the Kanamycin resistance gene and the pBR322 origin of replication (D). The recomb cloner plasmid was digested and recombined with the Ad7 genomic DNA in vitro using BJ5183 electrocompetent cells (E). The complete Ad7 genomic DNA plasmid is shown (F).
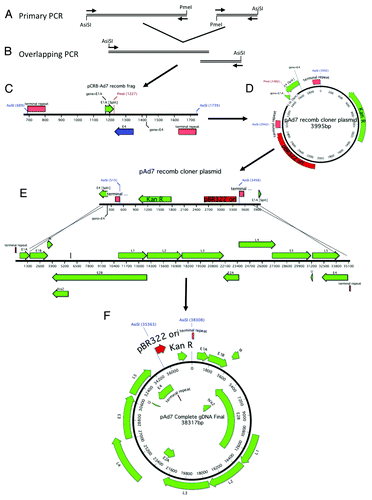
Table 2. Primers for Ad4 and Ad7 Shuttle Plasmids
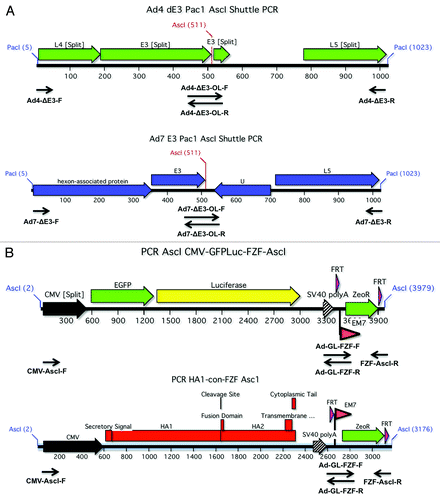
Figure 8. Construction of E3 shuttle plasmids and transgenes. In order to create replication-competent vectors, the nonessential E3 were genes replaced with either a HA1-con expression cassette or a GFPLuc fusion gene. The PCR products that were used to create the shuttle plasmids for Ad4 and Ad7 are shown (A). Both of the overlapping PCR products were designed to flank the E3 genes and contained unique AscI sites. The AscI flanked transgene expression cassettes are shown (B). The CMV expression cassettes were fused to a FRT flanked zeocin gene for selection of recombinants and ultimate removal by FLP recombinase.