Figures & data
Figure 1. Trial profile. Note: An issue was identified with the informed consent obtained for one child and the child’s parents didn’t permit GlaxoSmithKline Vaccines to use the child’s data. As a result, the data of the child, who had an SAE that was not considered to be related to the study medication by the investigator, are not detailed and were not used in the analysis.
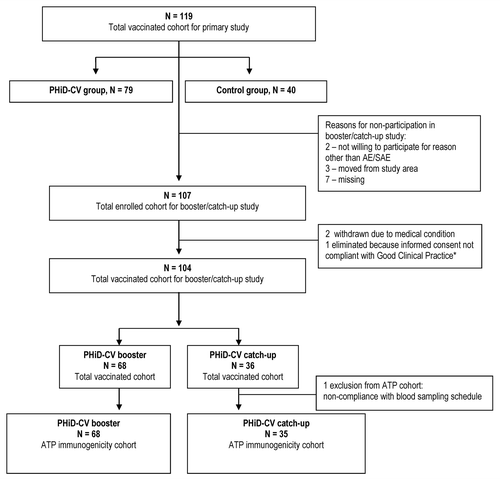
Table 1. Demographic characteristics (total vaccinated cohort)
Table 2. Incidences of solicited local and general symptoms per dose (total vaccinated cohort)
Figure 2. (A) Geometric mean antibody concentrations (GlaxoSmithKline’s 22F-inhibition ELISA, binary logarithmic scale, ATP cohort for immunogenicity) and (B) opsonophagocytic geometric mean titers against individual pneumococcal serotypes (decimal logarithmic scale, ATP cohort for immunogenicity). Note: Post-pri, 1 mo after 3-dose priming (at approximately 5 mo of age) with PHiD-CV in PHiD-CV booster group and control vaccine in PHiD-CV catch-up group; Pre-bst/catch-up, before booster dose in PHiD-CV booster group or before first catch-up dose in PHiD-CV catch-up group (15 to 21 mo of age); Post-bst/catch-up, 1 mo after booster dose in PHiD-CV booster group (16 to 22 mo of age) or 1 mo after second catch-up dose in PHiD-CV catch-up group (18 to 24 mo of age). Error bars represent 95% confidence intervals.
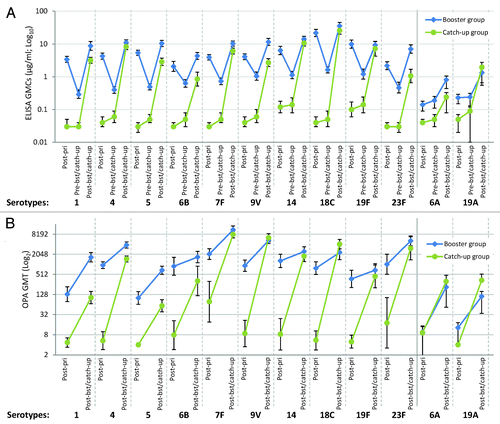
Table 3. 22F-ELISA antibody and opsonophagocytic responses against individual pneumococcal serotypes 1 mo after vaccination (ATP cohort for immunogenicity)
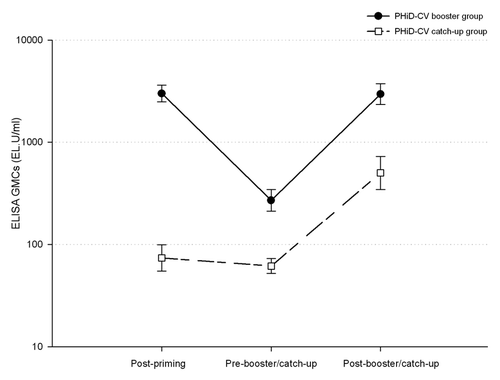
Figure 3. ELISA antibody responses (with 95% confidence intervals) against protein D (logarithmic scale, ATP cohort for immunogenicity). Note: Post-priming, 1 mo after 3-dose priming (at approximately 5 mo of age) with PHiD-CV in PHiD-CV booster group and control vaccine in PHiD-CV catch-up group; Pre-booster/catch-up, before booster dose in PHiD-CV booster group or before first catch-up dose in PHiD-CV catch-up group (15 to 21 mo of age); Post-booster/catch-up, 1 mo after booster dose in PHiD-CV booster group (16 to 22 mo of age) or 1 mo after second catch-up dose in PHiD-CV catch-up group (18 to 24 mo of age).