Figures & data
Figure 1. Immunologic characterization of humanized NOD/DR4/B7 dTg mice. (A) microsatellite analysis of the NOD genetic background in the parental NOD/DR4 Tg strain (upper panel). Comparison between the genetic background in 2 representative NOD wt, C57BL/6 parental strains, and 8 out of 32 microsatelites in the F12 generation of parental NOD/DR4 Tg strain shows full transfer of the NOD background in the parental NOD/DR4 Tg strain. Lower panel, identification of human HLA-DR*0401 and B7.1 (CD80) transgenes by PCR using specific primers (forward: GTTTCTTGGA GCAGGTTAAA CA; reverse: CTGCACTGTG AAGCTCTCAC, and respectively: forward: GCTTACAACC TTTGGAGACC CAG; reverse: CGTCACTTCA GCCAGGTG). Internal control PCR primers for DNA quantification were specific for mouse IgG3 gene (forward: ACAACAGCCC CATCTGTCTA T; reverse: GTGGGCTACG TTGCAGATGA C). Lane 1, DNA size markers; lanes 2–5, NOD mice expressing both the human HLA-DR*0401 and B7.1 transgenes; lanes 6–11, NOD/DR4 littermates lacking the hu B7.1 transgene. (B) expression of HLA-DR4 molecules on splenic APCs from NOD/DR4/B7 dTg mice. Left panel, splenic cells stained with a rat IgG isotype control Ab-FITC conjugate; Right panel, splenic cells stained with a rat IgG anti-HLA-DR4-FITC conjugate. Shown is the mean ± SD values as determined in 4 NOD/DR4/B7 mice. (C), FACS detection of GAD65271–285-specific CD4+ T-cells in the blood of NOD/DR4/B7 dTg mice. Left panel, the signal-to-noise background of the secondary anti-human IgG1-FITC conjugate; right panel, the mean frequency of GAD65271–280-specific CD4+ T cells ± SD measured in 4 NOD/DR4/B7 dTg mice in spleen cells double-stained with hu DEF-GAD65 reagent and revealed by a goat anti-human IgG1-FITC conjugate, and anti-mouse CD4 Ab-APC conjugate (clone #GK1.5, ATCC, BD PharMingen). (D) immunohistochemical detection of human B7.1 (CD80) expression in the pancreatic β-islets of NOD/DR4/B7 dTg mice. Fresh pancreatic sections of 5μm in OCT from a NOD wt mouse (left panel) and NOD/DR4/B7dTg mouse (right panel) were stained with a rat IgG anti-human B7 molecule (BD PharMingen) and revealed by an anti-rat IgG-HRP conjugate (Jackson ImmunoResearch). Shown is the absence of B7 staining of a representative β-islet from a NOD wt mouse, and the positive B7 staining for a representative β-islet from a NOD/DR4/B7 dTg mouse. Dark arrows in each panel indicate the position of pancreatic β-islets.
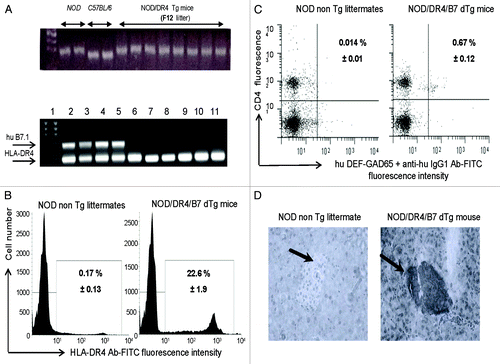
Figure 2. T1D incidence in the NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent. (A) Pre-diabetic, 2.5 mo-old NOD/DR4/B7 dTg mice were selected from 4 different litters (n = 14) and treated i.p. with 8 doses of 10 μg of hu DEF-GAD 65 reagent in saline (n = 14 mice) or with saline alone (control group, n = 9 mice) every other 4th day. Glycemia was measured bi-weekly from the tail vein. Y axis indicates the cumulative incidence of hyperglycemia calculated as a percent of mice developing hyperglycemia in each group at different time-points after treatment interruption. Grouped arrows on the X axis indicate the time-points and number of hu DEF-GAD65 i.p. injections. Shown is the significant relevance (* P value) between the two groups at the end of experiment. (B) hyperglycemia values in hu DEF-GAD65 non responders (NOD/DR4/B7 dTg mice) in two representative mice (Mouse #1 and #3) with stabilized hyperglycemia after one single dose of 20 μg hu DEF-GAD65 as followed up for 8 more months after injection. Arrows indicate the time of supplemental hu DEF-GAD65 injection (at 6 mo of age) that failed to reverse hyperglycemia in mice #1 and #3. Also shown is a NOD/DR4/B7 dTg mouse (mouse #2) treated with hu DEF-GAD65 reagent under the same regimen as in panel A, which developed mild hyperglycemia (250 and 270 mg/dL) some 1.5 mo after treatment interruption. Arrow indicates the time-point (6 mo of age) when mouse #2 received a supplemental 20 μg hu DEF-GAD65 i.p. injection that reversed hyperglycemia. Grouped arrows on the X axis indicate the time-points and number of hu DEF-GAD65 i.p. injections.
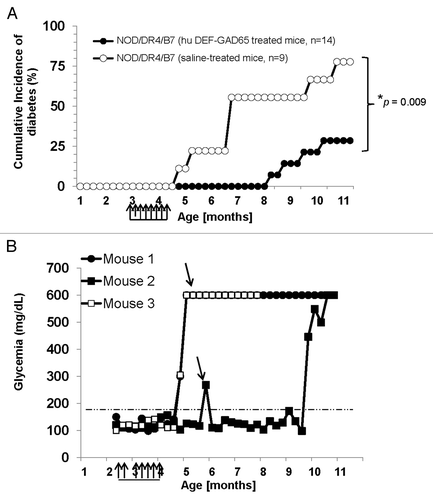
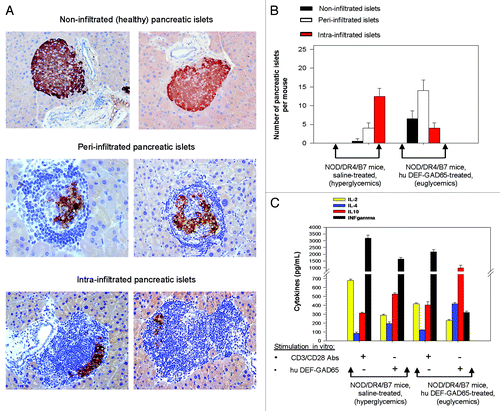
Figure 3. Pancreatic and T-cell analyses of NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent. (A) two representative, fully functional pancreatic β-islets lacking lymphocyte infiltration (upper panels), or peri-infiltrated β-islets (middle panels), or intra-infiltrated β-islets from the group of NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent as in , panel A. Pancreata from both groups of mice were analyzed after treatment interruption, when mice reached 11 mo of age. Shown in each panel are the HE staining of infiltrating lymphocytes (dark blue) and the presence of intra-islet insulin granules stained with a rabbit anti-Insulin-HRP conjugate. Of note, the peri-infiltrated islets show higher amount of insulin granules as compared with the intra-infiltrated islets. (B) Comparative morphologic analysis of pancreatic islet infiltration in NOD/DR4/B7 dTg mice treated or not with hu DEF-GAD65 reagent as in . The pancreata from both groups of mice were analyzed when mice reached 11 mo of age. Some 20–25 β-islets per pancreas were analyzed from individual mice (n = 5 mice/group). Of note, treated mice showed a significantly higher number of pancreatic peri-infiltrated islets than those in the control group. (C) cytokine analysis in stimulated spleen cultures from NOD/DR4/B7 dTg mice treated or not with hu DEF-GAD65 reagent. Of note, the CD4 T-cells from NOD/DR4/B7 dTg mice treated in vivo with hu DEF-GAD65 reagent secreted a significantly higher amount of IL-4 and IL-10, and lower amount of IFN-γ than those from saline-treated animals (control group). Shown are the mean values of cytokines ± SD for 4 individual mice in each group.