Figures & data
Figure 1. Mechanisms involved in immune escape in Multiple Myeloma. Multiple myeloma can inhibit efficient immune recognition and destruction via multiple direct and indirect mechanisms. These include impaired antigen presentation, signaling via co-inhibitory molecules, secretion of immunosuppressive factors and recruitment of suppressive immune cells. HLA, human leukocyte antigen; Th2 cell, T helper 2 cell; IDO, indoleamine 2,3-dioxygenase; TGF-β, transforming growth factor-β; sMIC-a, soluble major histocompatibility antigen class I polypeptide-related sequence A; IL-6, Interleukin-6; NK cell, natural killer cell; CTLA-4, cytotoxic T lymphocyte associated antigen-4; PD-1, programmed death-1; BTLA, B, and T lymphocyte attenuator.
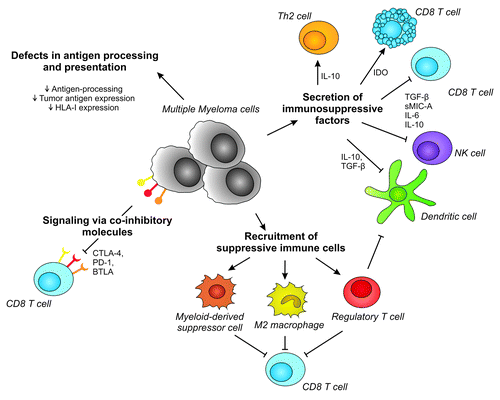
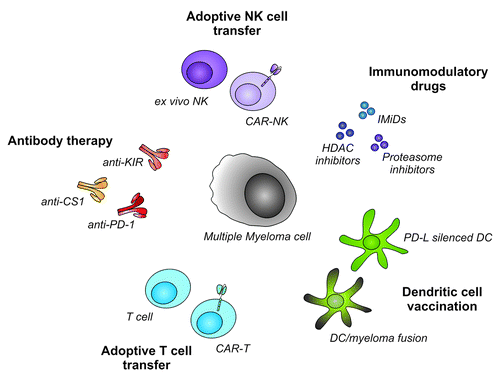
Figure 2. Cellular immunotherapeutic approaches in multiple myeloma. To improve survival in multiple myeloma patients, several cellular immunotherapies boosting NK cell and T cell mediated immunity, can be exploited. NK cells can be isolated or generated ex vivo, and can subsequently be used for adoptive transfer. The efficacy of NK cell-based therapy can be enhanced by the introduction of tumor-targeting CARs. Furthermore, combination with antibodies like anti-KIR or anti-CS1, or anti-myeloma drugs further boost anti-myeloma NK cell immunity. Adoptive T cell transfer after allogeneic stem cell transplantation can induce complete remission in MM patients. In addition adoptive transfer CAR-modified T cells might be even more specific and effective. Tumor specific T cells can also be expanded in vivo following DC vaccination. Silencing of co-inhibitory molecules like PD-L by silencing RNA, can further increase efficacy of DC vaccination. Finally, interference with co-inhibitory pathways using blocking antibodies like anti-PD1 is a promising strategy to increase the therapeutic effect. NK cell, Natural Killer cell; CAR, Chimeric Antigen Receptor; HDAC, Histone Deacetylase; PD-L, Programmed Death-ligand; DC, Dendritic Cell; anti-PD-1, anti-Programmed Death-1; anti-KIR, anti-Killer Immunoglobulin-like Receptor.