Figures & data
Figure 1. (A) Following intravaginal infection with HSV-2; stimulation with pathogenic symptomatic and protective asymptomatic human epitopes, expressed by the virus, greatly contributes to development of HSV-specific memory CD8+ T cell subsets, with either VM-resident effector memory (TEM) and tissue-resident memory (TRM) or lymphoid resident central memory CD8+ T cells (TCM). (See text for details). We hypothesize that virus derived symptomatic epitopes boost primarily CD8+ TCM, that reside primarily in the secondary lymphoid tissues, such as GT-DLN, which, once re-activated, re-circulate through the bloodstream into the vaginal mucosa (VM). In contrast, virus derived asymptomatic epitopes boost primarily VM-resident CD8+ TEM/TRM that are retained in the mucosal site of viral entry. (B). Anatomic-distribution, phenotypic, and functional characteristics of HSV-specific symptomatic central memory CD8+ T cells (TCM) vs. asymptomatic effector memory (TEM) CD8+ T-cells induced following intravaginal infection with HSV-2. Intra-vaginal (VAG) infection with HSV-2 bearing both symptomatic or asymptomatic epitopes prime naïve T cells into effector T cells to clear the virus infection while simultaneously generating memory CD8+ T cells to protect against subsequent encounter with the virus following re-infection or reactivation from latently infected sensory ganglia. We hypothesize that after the clearance of the virus, there is an optimum equilibrium between SLECs (IL7RlowKLRG1high) and MPECs (IL7RhighKLRG1low) cells derived from an early effector cells (EECs, IL7RlowKLRG1low), which then transit to 2 main categories of memory CD8+ T cells: TCM CD8+ T cells specific to symptomatic epitopes and TEM CD8+ T cells specific to asymptomatic epitopes. TEM CD8+ T-cells are mainly retained in the vaginal mucosa (see text for detail).
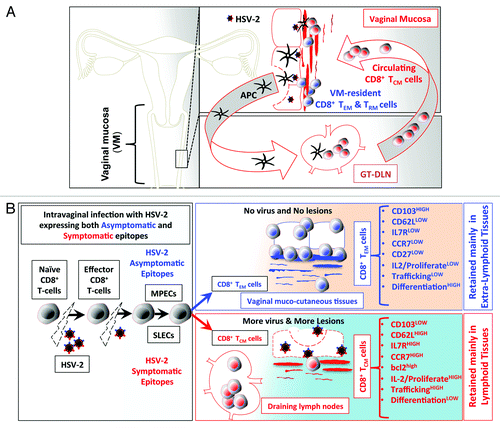
Figure 2. A proposed model of phenotypic and functional characteristics of HSV-specific CD8+ T cells in HSV-seropositive symptomatic vs. asymptomatic individuals: (A) Majority of women are asymptomatic and clear the virus from the VM by mounting a protective T cell responses while some women are symptomatic and develop genital herpes. Significant differences in the quantity, the quality and the durability of effector memory (TEM), tissue-resident memory (TRM) and central memory(TCM) CD8+ T cells specific to symptomatic vs. asymptomatic human HSV-2 epitopes are detected in symptomatic and asymptomatic women. (B) Potential interactions between an APC, presenting an asymptomatic vs. symptomatic epitope, (right) and a memory CD8+ T cell (left) occur in HSV-2 infected vaginal mucosa, and appear to be mediated by TCR/MHC, CD103/E-Cadherin, and CD127-IL7 pathways. (C) The balance between the asymptomatic vs. symptomatic epitopes will determine herpes protection vs. immunopathology.
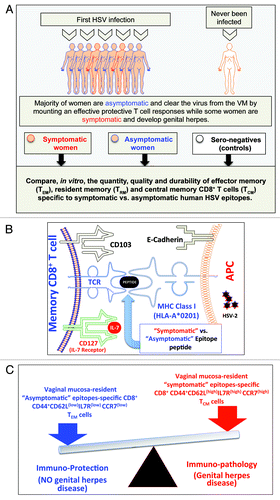
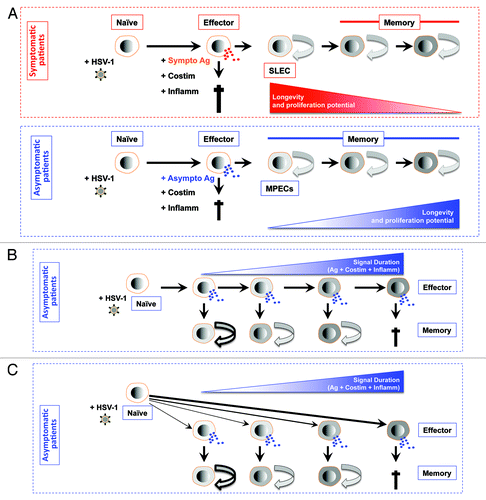
Figure 3. Models for generating diverse differentiated states of effector and memory CD8+ T cells following stimulation by symptomatic vs. asymptomatic epitopes. (A) First working model of de-differentiation for generating CD8+ T cell diversity proposes that soon after symptomatic or asymptomatic activation daughter naïve CD8+ cells become fully differentiated and functional by producing cytokines and exhibiting cytotoxic activity. The majority of effector CD8+ T cell then die. While a minority of asymptomatic epitope specific CD8+ T cells will de-differentiate into MPEC and long-lived memory CD8+ T cells. (B) Second working linear model for generating CD8+ T cell diversity proposes that degree of effector cell differentiation is regulated by the duration of exposure of daughter cells to symptomatic and asymptomatic epitopes following repetitive reactivation of HSV-2 from latency. The majority of terminally differentiated effector CD8+ T cell will die following cumulative encounter with the virus. While a minority of asymptomatic epitope specific CD8+ T cells which did not reach the end stage of differentiation will develop into MPEC and long-lived memory CD8+ T cells. The memory CD8+ T cell phenotypes vary according to the differentiation state of the effector cells from which they descended; curved arrows with bold, thin, or dashed lines indicate a high, medium, or low degree of proliferative potential and longevity. (C) Third working divergent model for generating CD8+ T cell diversity is similar to B, except that the strength of symptomatic vs. asymptomatic signal (instead of the cumulative symptomatic vs. asymptomatic stimulation) will determine the degree of effector cell differentiation and formation MPEC and long-lived memory CD8+ T cells.