Figures & data
Table 1. Baseline HPV serostatus and HPV-DNA status at time of inclusion in the study
Table 2. Geometric mean antibody titers (GMTs) and GMT ratios according to baseline seropositivity and anogenital human papillomavirus (HPV)
Figure 1. Seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies in baseline HPV-negative cohorts. Seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per vaccine, per timepoint. Percentages indicate seropositivity rates at each timepoint (seropositivity defined as PBNA ED50 > 40). HPV, human papillomavirus; GMTs, geometric mean titers.
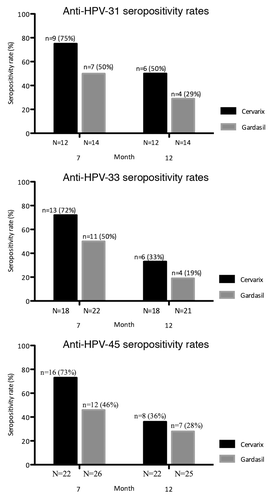
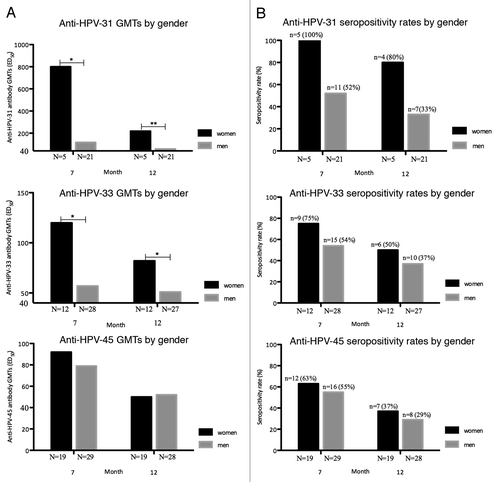
Figure 2. Gender-specific geometric mean titers (GMTs) and seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies in baseline HPV-negative cohorts. (A) Gender-specific geometric mean antibody titer computed using the log10 transformation of titers as measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects. *P < 0.05, **P < 0.001. HPV, human papillomavirus; GMTs, geometric mean titers. (B) Gender-specific seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per gender, per timepoint. Percentages indicate seropositivity rates at each timepoint (seropositivity defined as PBNA ED50 > 40). HPV, human papillomavirus; GMTs, geometric mean titers.