Figures & data
Figure 1. CONSORT diagram showing disposition of study participants. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
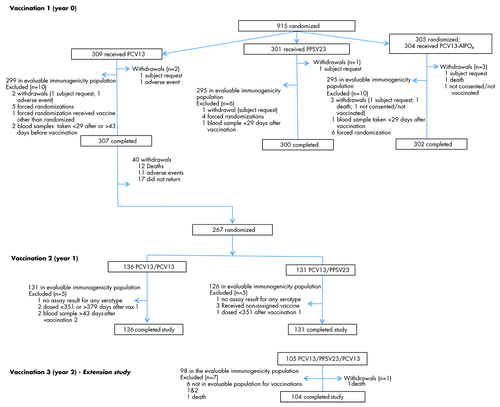
Table 1. Participant demographics of the evaluable immunogenicity population
Table 2. Pneumococcal IgG GMCs 1 mo after PCV13 without or with AlPO4 (evaluable immunogenicity population)
Table 3. Pneumococcal immune responses 1 mo after PCV13 and PPSV23 (evaluable immunogenicity population)
Table 4. Immune responses 1 mo after vaccine sequence PCV13/PPSV23 given at a 1-y interval compared with after a single dose of PPSV23 (evaluable immunogenicity population)
Table 5. Immune responses 1 mo after dose 1 and dose 2 of PCV13 (evaluable immunogenicity population)
Table 6. Immune responses after each vaccination in the vaccine sequence PCV13/PPSV23/PCV13
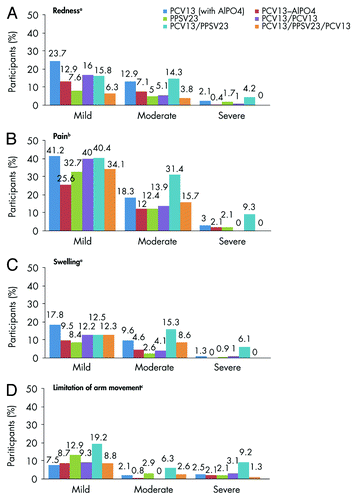
Figure 2. Local reactions reported within 14 d after vaccination. aSeverity of redness and swelling: absent (no or minimal redness/swelling present, 0 to <2.5 cm; 0 to <5 caliper units), mild (2.5–5.0 cm; ≥5 to ≤10 caliper units), moderate (5.1–10.0 cm; >10 to ≤20 caliper units), or severe (>10.0 cm; >20 caliper units). bSeverity of pain at the injection(s) site: mild (awareness of sign/symptom but easily tolerated), moderate (enough discomfort to interfere with usual activity), or severe (inability to do usual activity).cSeverity of limitation of arm movement: absent (no limitation), mild (some limitation), moderate (able to move arm above shoulder-level but not above head), or severe (unable to move arm above shoulder). Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.