Figures & data
Figure 1. Flow of participants through the trial. 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 40/40, 40 μg each of HPV-16 and -18 L1 virus-like particles; ATP, according-to-protocol; M, month. This article focuses on subjects randomized to the 3D 20/20 M0,1,6 group and the 2D 20/20 M0,6 group (shaded boxes). Disposition data are also shown for subjects enrolled in other study groups (2D 40/40 M0,6 and 2D 40/40 M0,2) for completeness.
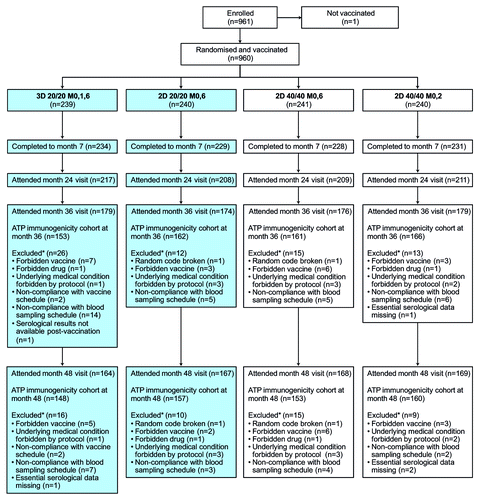
Table 1. HPV-16 and HPV-18 antibody responses up to month 48 in subjects aged 9–25 y (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline)
Table 2. HPV-16 and HPV-18 GMT ratios for 3D schedule in women aged 15–25 y over 2D schedule in girls aged 9–14 y at months 36 and 48 (according-to-protocol month 36 and 48 immunogenicity cohorts, subjects seronegative at baseline)
Figure 2. Kinetics of HPV-16 and HPV-18 antibody responses for girls aged 9–14 y in the 2D 20/20 M0,6 group and women aged 15–25 y in the 3D 20/20 M0,1,6 group (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline). 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month. Natural infection, GMT in subjects who had cleared a natural infection.Citation12 Plateau, GMT at the plateau level (month 45–50) in women aged 15–25 y (total vaccinated cohort) in a study in which sustained protection with the HPV-16/18 AS04-adjuvanted vaccine has been shown (i.e., 397.8 EU/mL for HPV-16 and 297.3 EU/mL for HPV-18).Citation13
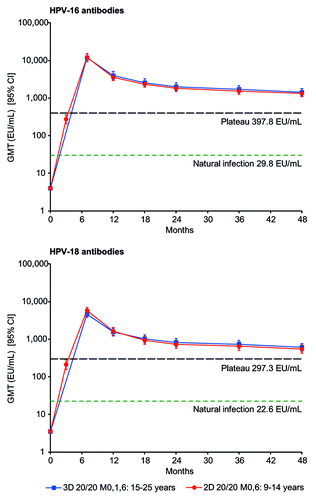
Table 3. HPV-31 and HPV-45 antibody responses for the 3D schedule in women aged 15–25 y and the 2D schedule in girls aged 9–14 y up to month 48 (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline)
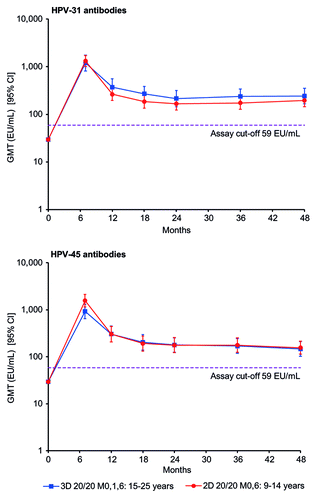
Figure 3. Kinetics of cross-reactive HPV-31 and HPV-45 antibody responses for girls aged 9–14 y in the 2D 20/20 M0,6 group and women aged 15–25 y in the 3D 20/20 M0,1,6 group (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline). 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month.