Figures & data
Figure 1. Preparation of the experimental inactivated CA16 vaccine and its characterization. (A) Purification of experimental inactivated CA16 vaccine in bulk and measurements of the antigen distribution. The inactivated CA16 bulk was purified through Sepharose 6FF; samples were collected every minute until the baseline became stable. The profile of the viral antigen titers for each sample (1 mL per sample) was measured using an ELISA assay with anti-CA16 polyclonal antiserum. (B) Electron microscopy images showing the antigen peak distributed in the second fraction containing virions. The black arrow indicates the complete viral particles (solid virion), and the white arrow indicates the virion-containing capsids without RNA (empty virion). The bar represents 100 nm. (C) SDS-PAGE analysis of the bulk-produced experimental vaccine. The bulk-produced experimental vaccine was analyzed on 12% polyacrylamide gels via silver staining. (D) Western blotting with anti-CA16 antibody. The CA16 antigens were detected via western blot using rabbit anti-CA16 sera.
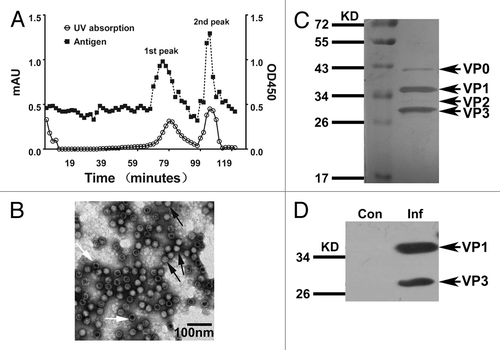
Figure 2. The dynamic profile and cross-neutralization reactivity for neutralizing antibody induced using immunization with the experimental inactivated CA16 vaccine in mice. (A) Neutralizing antibody dynamic profile. Neutralization titers (GMTs) of the serum from immunized mice via the 400 EU (n = 8), 200 EU (n = 8), 100 EU (n = 8), 50 EU (n = 8), 25 EU (n = 8), and 12.5 EU (n = 8) of inactivated CA16 vaccine or PBS (control, n = 8) collected at day 28, 35, 42, 70, and 84 after the first immunization were determined via neutralization test at the highest dilution that prevented the cells from experiencing cytopathic effects. Each symbol represents a dose, and the line indicates the variation of the antibody in the group. (B) Cross-neutralization test. The cross-neutralization of the serum was detected with the G20, G10, and MA154 virus strains, respectively. The neutralization titers (GMTs+95%CI) of the sera from immunized mice via 400 EU (n = 8), 200 EU (n = 8), 100 EU (n = 8), 50 EU (n = 8), 25 EU (n = 8), and 12.5 EU (n = 8) of the inactivated CA16 vaccine or PBS (control, n = 8) collected at day 70 after the first immunization were determined using a neutralization test at the highest dilution that prevented the cells from experiencing cytopathic effects.
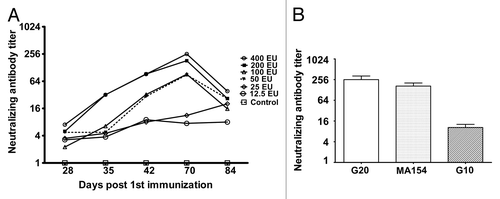
Figure 3. The immune protective efficacy of the candidate inactivated CA16 vaccine against infection with different challenge viruses in a neonatal passive immunity mouse model. Three groups of suckling mice (24–48 h after birth) obtained from the immunized maternal mice of each dosage group after the secondary immunization were injected intracerebrally with either CA16 virus G-20, MA154, or G10 (104CCID50 per mouse), and the control group from the parents was injected with the 20 µl Al(OH)3 adjuvant. Subsequently, the survival rates were recorded daily after infection for a period of 15 d. (A) G20 challenge group: 400 EU x 2n (n = 11–12), 200 EU × 2n (n = 12–13), 100 EU × 2n (n = 10–12), 50 EU × 2n (n = 12–15), 25 EU × 2n (n = 11–12), 12.5 EU × 2n (n = 12–13), n.c (negative control) × 2n (n = 11–12). 2n indicates the number (n) of mice in 2 replicates of a dose group. (B) G10 challenge group: 400 EU × 2n (n = 12–13), 200 EU × 2n (n = 10–11), 100 EU × 2n (n = 10–11), 50 EU × 2n (n = 12–15), 25 EU × 2n (n = 11–16), 12.5 EU × 2n (n = 13–15), n.c × 2n (n = 10–12). (C) MA154 challenge group: 400 EU × 2n (n = 10–11), 200 EU × 2n (n = 12–13), 100 EU × 2n (n = 10–13), 50 EU × 2n (n = 12–13), 25 EU × 2n (n = 11–15), 12.5 EU × 2n (n = 10–13), n.c × 2n (n = 12–14)
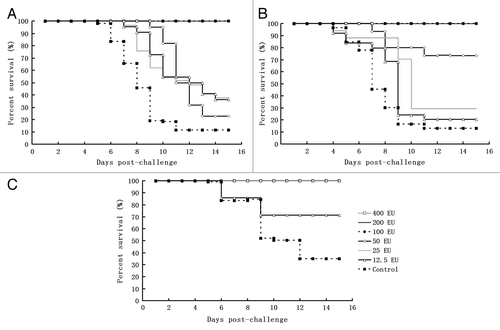
Figure 4. The dynamic profile and cross-neutralization reactivity for neutralizing antibody induced via immunization with the experimental inactivated CA16 vaccine in rhesus monkeys. (A) Neutralizing antibody dynamic profile. Neutralization titers (GMTs + 95%CI) of the sera collected from immunized rhesus monkeys via the 1000 EU (n = 4), 600 EU (n = 4), and 200 EU (n = 4) of inactivated CA16 vaccine or PBS (control, n = 4) at day 28, 35, 42, 56, 63, and 70 after the first immunization were determined via a neutralization test based upon the highest serum dilution that prevented cells from experiencing cytopathic effects. Each symbol represents a dose, and the line indicates the geometric mean titer (GMT) of the group. (B) Cross-neutralization reactivity. The cross-neutralization antibody titers were detected with G20, G10, or MA154. Neutralization titers (GMTs + 95%CI) of the sera from immunized rhesus monkeys via the 1000 EU (n = 4), 600 EU (n = 4), and 200 EU (n = 4) of inactivated CA16 vaccine or PBS (control, n = 4) collected at day 42 after the first immunization were determined via a neutralization test at the highest dilution that prevented the cells from experiencing cytopathic effects.
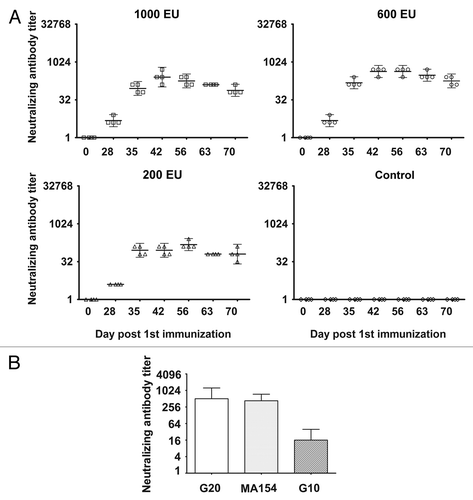
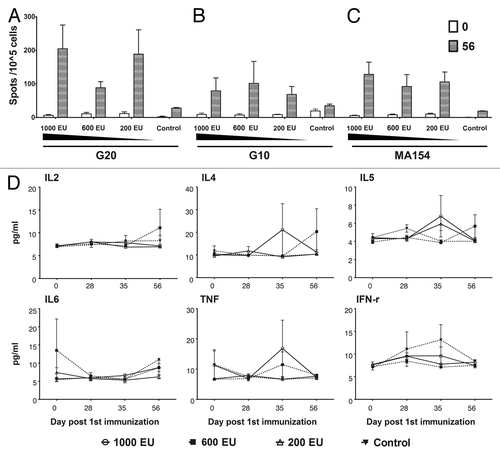
Figure 5. Characterization of immune responses induced in rhesus monkeys by the candidate inactivated CA16 vaccine. Four groups of rhesus monkeys were subcutaneously immunized with CA16 vaccines of different doses and Al(OH)3 adjuvant as described in the Figure S1 legend. PBMCs were collected on 0 and 56 d after first immunization and analyzed with the IFN-γ Elispot Kit. The pro-inflammatory factors of the sera collected at same time points were measured with a CBA Kit as described in Materials and Methods. (A) Stimulation with the G20 viral strain. (B) Stimulation with the G10 viral strain. (C) Stimulation with the MF154 viral strain. (D) Measurement of pro-inflammatory cytokine levels. The data are shown as means ± SD. Two replicates were performed for each sample.