Figures & data
Figure 1. Proliferative PBMC responses in RA patients. PBMC from 19 RA patients were screened for proliferative responses to cartilage antigen before and 2 y after treatment. Open bars (PBMC), cell culture medium; closed bars (PBMC+AG), cartilage antigen. y-axis, results of thymidine uptake assay (means ± SEM counts per minute).**P < 0.01, significant differences between groups before and after therapy (U criterion).
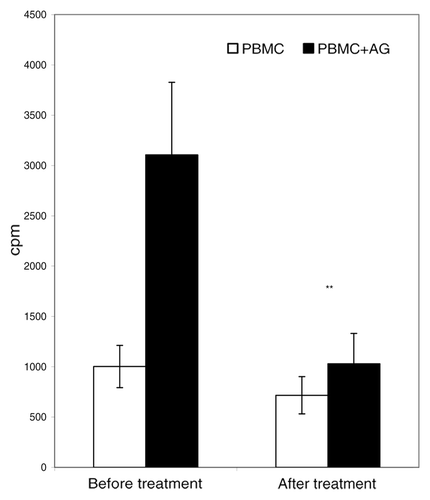
Figure 2. Concentration of IFNγ and IL-4 in sera of patients in the course of the T-cell vaccination. Concentrations of IFNγ (open bars) and IL-4 (closed bars) were measured in sera from 12 RA patients before and after T-cell vaccination. P < 0.05; **P < 0.01, as compared with the level before therapy onset (U criterion). Error bars denote means ± SEM (cytokine concentrations).
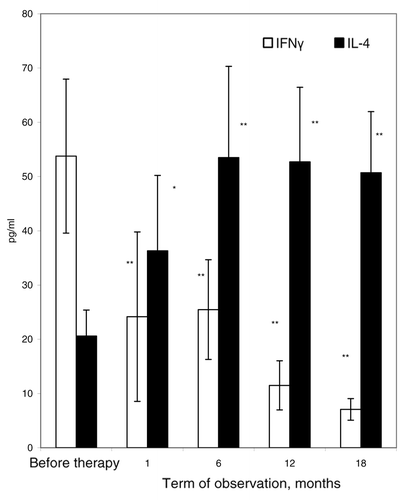
Figure 3. Concentrations of IFN-γ and IL-4 in cell culture supernatants of PBMC from RA patients. PBMC samples (n = 12) from RA patients were taken before (open bars) and 2 y after (closed bars) onset of T-cell vaccination. PBMC were incubated in the absence of antigens (1) or in the presence of PHA (2) or of the protein cartilage antigen (3) for 72 h. Concentrations of IFN-γ (A) and IL-4 (B) in cell culture supernatants were analyzed by ELISA. *P < 0.05; **P < 0.01, as compared with the cytokine levels before therapy onset (U criterion). Error bars denote means ± SEM (cytokine concentrations).
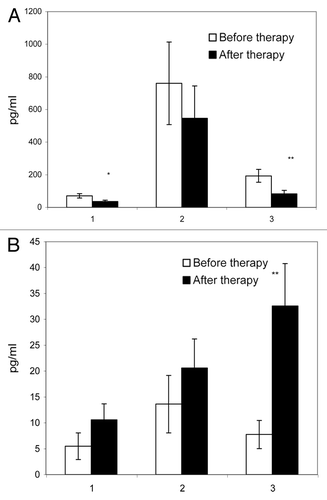
Figure 4. Percentages of IFN-γ and IL-4 – producing CD4 and CD8 lymphocytes in peripheral blood of RA patients before and after induction course of T-cell vaccination. PBMC from 7 RA patients were analyzed by flow cytometry for IFN-γ and IL-4 production in CD4 and CD8 T-cell populations before (closed bars) and after induction course of T-cell vaccination (open bars). The y-axis shows percentage of IFN-γ- and IL-4-producing CD4+ and CD8+ T-cells from the total number of lymphocytes. **P < 0.01, as compared with the amount of cytokine-producing lymphocytes before therapy onset (paired Wilcoxon criterion). Error bars denote the means ± SEM (percentage of positive cells).
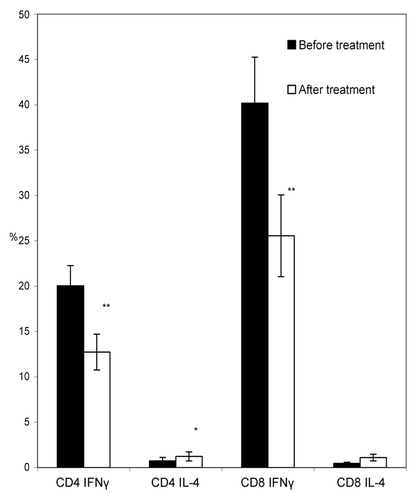
Table 1. Memory T-cell content in RA patients in the course of T-cell vaccination (M ± m)
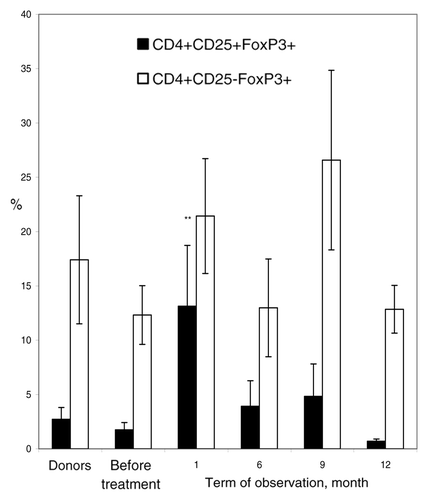
Figure 5. Percentage of regulatory T-cells in the course of T-cell vaccination in RA patients. PBMC from RA patients (n = 11) were analyzed by flow cytometry for “naïve” regulatory CD4+CD25+FoxP3+ T-cells (closed bars) and induced regulatory CD4+CD25-FoxP3+ T-cells (open bars) before the onset and in course of T-cell vaccination (observation period 12 mo), as compared with PBMC from healthy donors (n = 10). Percentage of positive cells from the total lymphoid cell population (y-axis) was determined by flow cytometry. **P < 0.01, as compared with the levels before therapy (U criterion). Error bars denote means ± SEM (percentage of positive cells).