Figures & data
Table 1. Demographic characteristics and underlying high-risk diseases, overall and stratified by age
Table 2. Hemagglutination-inhibition responses to 2011–2012 trivalent inactivated influenza vaccine antigens, overall and stratified by age group
Table 3. Geometric mean titers for 2011–2012 influenza A vaccine and selected historical strains, overall and stratified by age
Figure 1. Percent of participants with seroprotective (titer ≥ 1:40) at Day 0 to A(H1N1) and A(H3N2) influenza virus strains. The overall height of the bars indicates the percent of participants with seroprotective titers (HI ≥ 1:40) to a given historical strain at Day 0. The upper part of each bar indicates the percent of those individuals who seroconverted (≥4-fold rise in titer) against the specified current influenza vaccine A virus strain. *Difference in percent seroprotected between age groups (P < 0.05). A/PR/8/1934 = A/Puerto Rico/8/1934; A/FM/1/1947 = A/Fort Monmouth/1/1947; A/TX/36/1991 = A/Texas/36/1991; A/NC/1999 = A/New Caledonia/20/1999; A/SI/2006 = A/Solomon Islands/3/2006; A/Bris/59/2007 = A/Brisbane/59/2007; A/CA/07/2009 = A/California/07/2009; A/Syd/5/1997 = A/Sydney/5/1997; A/Pan/2007/99 = A/Panama/2007/1999; A/Fuj/411/2002 = A/Fujian/411/2002; A/Wis/67/2005 = A/Wisconsin/67/2005; A/Bris/10/2007 = A/Brisbane/10/2007.
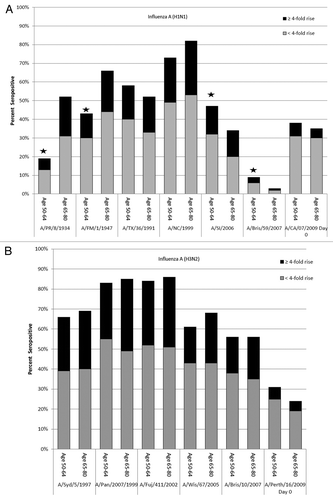
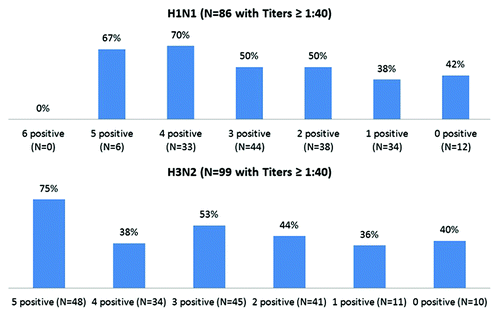
Figure 2. Proportion of participants with seroprotected (titers ≥ 1:40) at Day 21 by type and by the count of seropositive historical influenza strains among those seronegative at Day 0. The number of historical viruses for which each specimen had a titer ≥ 1:40 was called the “historical count”—for example, if a given serum specimen were tested against 6 historical viruses and had a titer ≥ 1:40 for 4 of them, the historical count was 4.