Figures & data
Figure 1. Summary of the trial design. * A dose:3.50 ± 0.25 logCCID50/ml, B dose:4.25 ± 0.25 logCCID50/ml, C dose:5.00 ± 0.25 logCCID50/ml. (A) Adult: 16 y-old~ < 60 y-old. (B) Child: 5 y-old~ < 16 y-old. (C) Preschool: 2 y-old~ < 5 y-old. (D) Infant: 8 mo-old~ < 2 y-old.
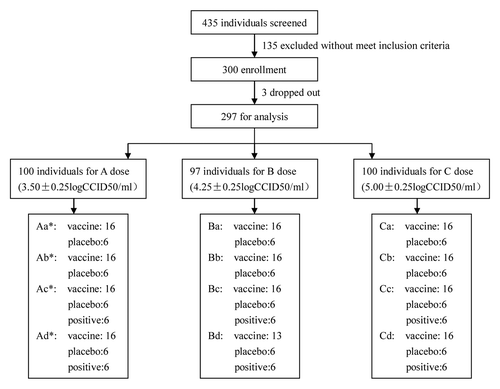
Table 1. The description of baseline level before immunization
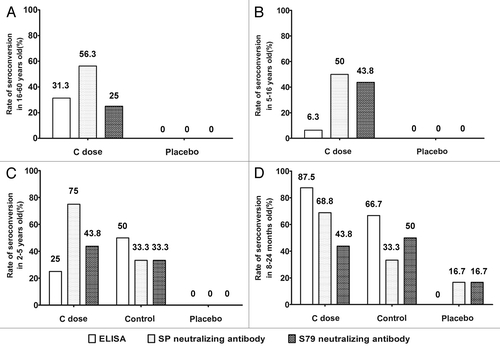
Figure 2. Seroconversion rates of serum 28 d after vaccination in C dose, placebo and control groups. Containing individuals (A) 16–60 y old, (B) 5–16 y old, (C) 2–5 y old and (D) 8–24 mo old. Detection of antibody, including the neutralizing antibodies against SP-A virus and S79 virus, and the antibody detected by ELISA. The genotype A vaccine control group was added in the 2–5-y-old and 8–24-mo-old groups. Seroconversion definition: ELISA:An antibody titer > 12 U/ml was considered seropositive. Neutralizing antibody: An antibody titer > 1:4 was considered seropositive.